How big is a sodium atom?
Thursday 31 July 2014
The new IB data booklet which is available on the OCC has some very different data in it for atomic (and ionic) radii compared to previous versions.
For example:
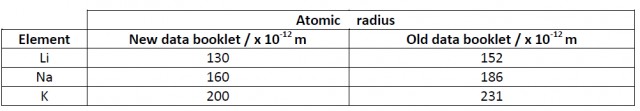
 It looks as if the radius of sodium atoms, for example, has shrunk considerably (from 186 x 10-12 m to 160 x 10-12 m, i.e. by 14%) during the past few years! The main source given in both data booklets is the CRC handbook although different editions have been used. The current (2014) data booklet uses the 2012 edition[1] whereas the 2009 data booklet uses the 2008 edition.
It looks as if the radius of sodium atoms, for example, has shrunk considerably (from 186 x 10-12 m to 160 x 10-12 m, i.e. by 14%) during the past few years! The main source given in both data booklets is the CRC handbook although different editions have been used. The current (2014) data booklet uses the 2012 edition[1] whereas the 2009 data booklet uses the 2008 edition.
The problem is that the IB data booklet does not state which radius it is quoting. Since the source has not basically changed it would appear that whoever put the new data booklet together has chosen to use a different way of arriving at the value.
The definition of atomic radius - the distance from the centre of the nucleus to the outermost electron - is by its very nature impossible to measure as the exact position of the electron cannot be known at any one moment in time. This means that there are various ways in which atomic radii are determined.
In general there would appear to be three main ways in which different radii which are often quoted, the metallic, covalent and van der Waals’ radius although the website Webelements quotes even more different ways and gives a definition of each way together with the stated value.
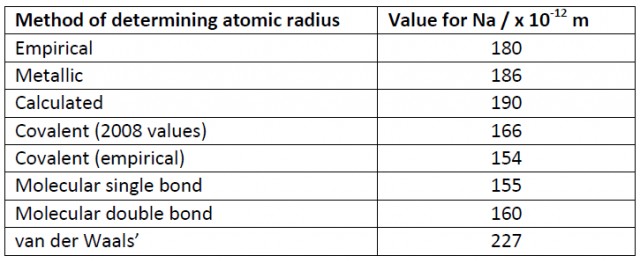
What appears to have happened is than in previous IB data booklets the metallic radius has been given for sodium whereas in the current data booklet the covalent radius has been chosen. Probably it does not really matter but maybe it is a good example of the fickle Nature of Science in action!
Footnotes
- 1. Haynes, WM, (ed). 2012. CRC Handbook of chemistry and physics. (93rd edition). Boca Raton, US. CRC Press.


Comments
To post comments you need to log in. If it is your first time you will need to subscribe.