Training students to think critically
Thursday 8 September 2022
 Young children are naturally curious and parents and teachers should actively encourage this curiosity. Questions which generally start with "How.." often require factual answers (e.g. How does this work? or How can I make this?) whereas questions which start with "Why..." often require more challenging answers. Critical thinking is perhaps a step up from curiosity as it generally requires the thinker to draw on the knowledge they possess from a different perspective. By the time students embark upon their IB Diploma chemistry course they may already have been schooled to accept certain ways of explaining basic chemistry and much of their innate curiosity may have atrophied or sadly been drilled out of them. We may need to stimulate them to question what they learn and not just accept information that is contradictory or incomplete whatever the source. This is partly because in their early years chemistry is often presented in a simplified manner and students then retain the wrong (or highly simplified) model which can hinder their understanding as they study chemistry at a more advanced level. Even for Diploma chemistry we still need to simply the explanations we give but a good teacher can at least make students aware of the flaws in the models used by inspiring them to look at them critically.
Young children are naturally curious and parents and teachers should actively encourage this curiosity. Questions which generally start with "How.." often require factual answers (e.g. How does this work? or How can I make this?) whereas questions which start with "Why..." often require more challenging answers. Critical thinking is perhaps a step up from curiosity as it generally requires the thinker to draw on the knowledge they possess from a different perspective. By the time students embark upon their IB Diploma chemistry course they may already have been schooled to accept certain ways of explaining basic chemistry and much of their innate curiosity may have atrophied or sadly been drilled out of them. We may need to stimulate them to question what they learn and not just accept information that is contradictory or incomplete whatever the source. This is partly because in their early years chemistry is often presented in a simplified manner and students then retain the wrong (or highly simplified) model which can hinder their understanding as they study chemistry at a more advanced level. Even for Diploma chemistry we still need to simply the explanations we give but a good teacher can at least make students aware of the flaws in the models used by inspiring them to look at them critically.
Article in Education in Chemistry
The Royal Society of Chemistry has just published an article in Education in Chemistry on "How to draw dot and cross diagrams" for teaching chemistry to the 14-16 (i.e. pre-IB) age group. Using magnesium oxide and aluminium oxide as examples, it shows students how to draw ionic bonds using the 'dot and cross' method following the octet rule. It includes a poster to download and display in the classroom, plus a fact sheet, a student worksheet and teacher notes for the stepwise process.
Using a marker pen to complete the re-usable RSC worksheet inside a plastic wallet
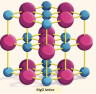
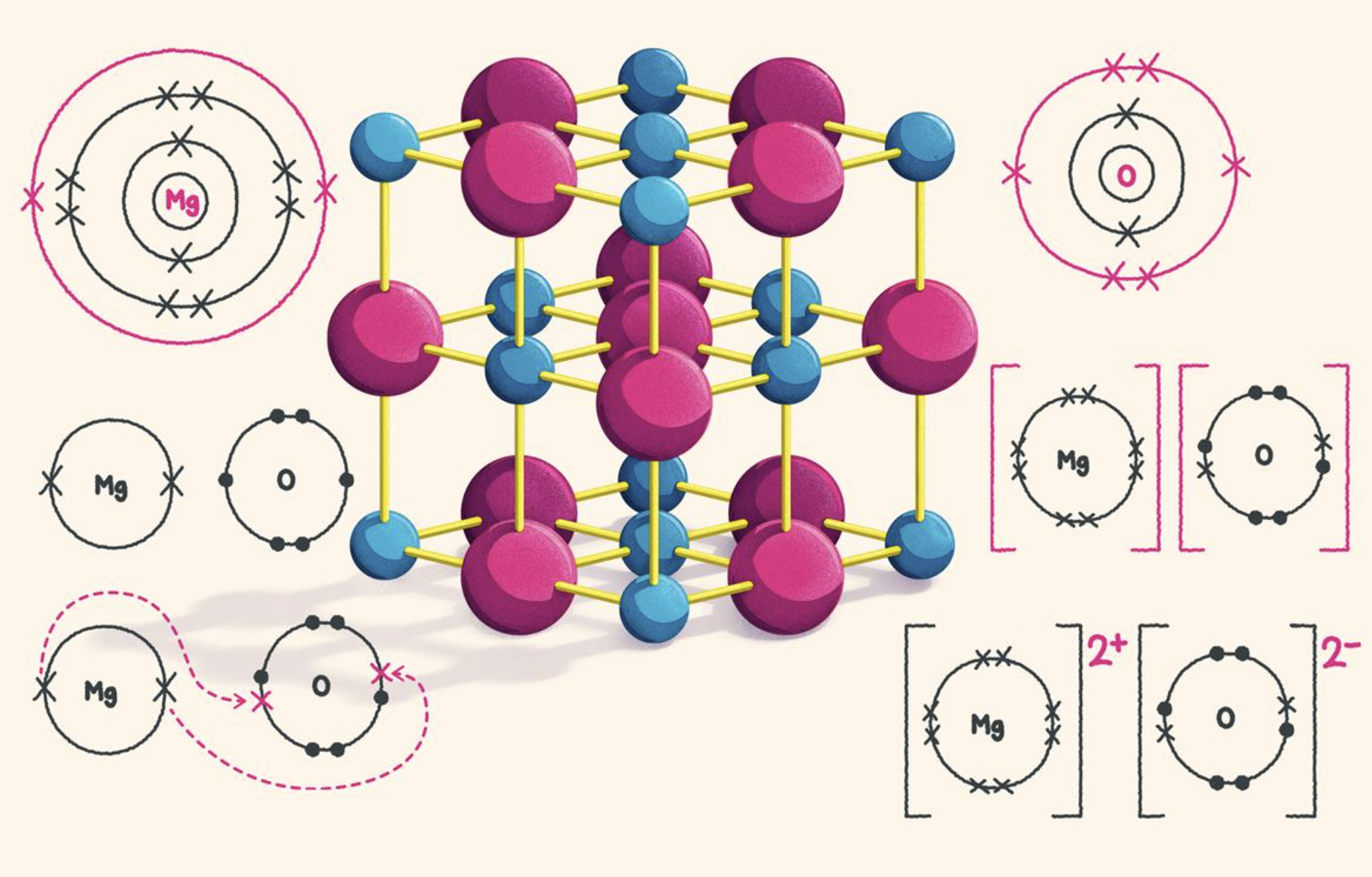
All good stuff but the model could be vastly improved to make it more accurate even for this age group by challenging them to criticise it using knowledge they already possess, or could easily deduce. This would then put them in a much stronger position when they come to learn Diploma level chemistry such as lattice enthalpy and the Born-Haber cycle. For example, look at the poster which shows magnesium and oxygen atoms combining to form magnesium oxide.
The RSC poster
It contains a nice colourful model of part of the magnesium oxide lattice but the model does not actually correspond to the 'dot and cross' diagrams shown. Teachers should challenge their students to look critically at the poster to see what inconsistencies they can detect. Hopefully students will spot one or more, but, even if they do not, you could then point out that one of the two different types of ions is much smaller than the other type in the colourful model and yet they are both shown the same size in the dot and cross diagrams. Pre-IB chemistry includes the composition of atoms in terms of protons, neutrons and electrons so students should easily deduce (as the diagram shows) that when magnesium atoms lose electrons the ion formed will be smaller but they should also be able to deduce what the diagram does not show, namely that the O2− ion will be much larger than an O atom. So that, even though they both have the same number of electrons, O2− ions have a much larger radius than Mg2+ ions as oxygen has four less protons in its nucleus than magnesium. Students should understand that obtaining a "complete outer shell" does not really explain anything. It is just a useful tool to show how the electrons are arranged in ionic compounds. The ionic bond in magnesium oxide is not just the attraction between one positive magnesium ion and one negative oxygen. It is actually the sum of all the positive and negative electrostatic attractive forces between the ions in the lattice. To break the lattice requires energy to overcome the sum of all these attractions and repulsions hence the high melting point of magnesium oxide and ionic compounds in general. The reverse process, the formation of the ionic lattice, gives out the same amount of energy. This is the source of the energy required to remove electrons from magnesium atoms to form magnesium ions (and to add two electrons to each oxygen atom). This leads on to the idea that the "shells" are actually energy levels. None of this involves chemistry that is not covered by most 14-16 syllabuses and it provides a much better basis for going on to Diploma level. Of course Diploma students should be able to see another glaring problem with the model shown and that is that the distance between the different energy levels. The levels should be shown getting closer together as they get further from the nucleus. You can read much more about this (and other inconsistencies in the model) on my Ionic bonding & structure page and also get your students to do the Data response question on ionic bonding. There is much more on critical thinking with examples on the pages Critical thinking and NOS & Critical Thinking.



Comments 2
Reading this some would say that there is a fine line between pedantry and critical thinking. But seriously, this would make a great starter for a class on ionic radii as in spotting the mistakes on the poster.
Great words as always Geoff. Bonding is one of the most misunderstood of topics pre and post 16. The many stumbling blocks include; the expanded and contracted octet, formal charge as an accounting tool, through to predicting possible Lewis Structures. All steps on a road that ultimately leads to questions of what actually is an electron ? where is it ! A great JCE article here on this very topic pubs.acs.org
To post comments you need to log in. If it is your first time you will need to subscribe.