Thinking critically
Saturday 19 February 2011
How can we get our students to think critically? Often there seems to be so little time just to cover what is on the IB Chemistry syllabus let alone get them to think and criticise what they read or are told. By the time they reach age 16 or 17 much of their innate curiosity seems to have been drummed out of them by the demands of taking one exam after another. I think we can no longer rely on students to think critically unaided. We need to show them how whenever the opportunity arises.
I’ll try to illustrate this with just one example. I’ve recently written the webpage on nucleophilic addition to cover sub-topic 2 in Option G. Superficially it is very straightforward. Just one reaction really – the addition of hydrogen cyanide to aldehydes and ketones. All the text books, websites etc. make it look so simple.
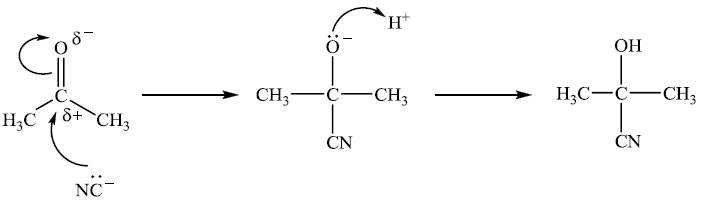
The carbon atom of the C=O group is δ+ as oxygen is more electronegative than carbon. So cyanide ions, CN-, are attracted to it to form an intermediate anion with the negative charge on the oxygen. This anion then picks up H+ to form the cyanohydrin product. This sums up pretty much all there is about this in the text books. All students have to do is learn it, answer the questions by essentially repeating this in the Paper 3 examination and they are on their way to achieving grade 7. But is it really this simple?
Firstly consider the nucleophile. The topic actually says the nucleophilic addition of hydrogen cyanide not cyanide ions. Why can’t hydrogen cyanide itself act as the nucleophile?
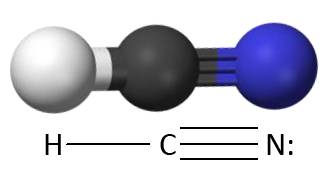
Like water and ammonia, two nucleophiles that students have already come across when studying nucleophilic substitution, hydrogen cyanide is also a polar molecule with at least one non-bonding pair of electrons. A student might reasonably expect the non-bonding pair of electrons on the nitrogen atom to be attracted to the carbonyl carbon atom (similar to what happens with ammonia when it substitutes in halogenoalkanes). Clearly this doesn’t happen – why not?
Now consider the cyanide ion which does act as the nucleophile. It actually contains two non-bonding pairs of electrons. One on the carbon atom, and one on the nitrogen atom (see below). Nitrogen is more electronegative than carbon so it would seem reasonable that it would be the more negative nitrogen atom that would preferentially approach the carbonyl δ+ carbon atom to form a C-N bond but this does not happen. Why not?
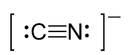
![]()
You can extend this to look at how a cyanide ion can act as a ligand with transition metals. Again its Lewis base properties depend upon the non-bonding pair of electrons on the carbon atom not the more electronegative nitrogen atom. It is the carbon atom that forms the coordinate bond to the transition metal as in iron(III) hexacyanide, [Fe(CN)6]3+.
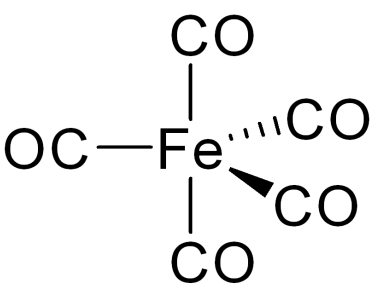 An analogous situation arises with carbon monoxide which is isoelectronic (contains the same number of electrons) to the cyanide ion. It too has two non-bonding pairs of electrons (see above). Why isn’t carbon monoxide a good nucleophile – the carbon atom and the oxygen atom both have one non-bonding pair of electrons they could use to approach a δ+ carbon atom? After all it is a good ligand and like cyanide ions when it acts as a Lewis base with transition metals again it is the non-bonding pair on the carbon atom, not the more electronegative oxygen atom, which forms the coordinate bond (as, for example, is shown in the image on the left for iron pentacarbonyl, Fe(CO)5).
An analogous situation arises with carbon monoxide which is isoelectronic (contains the same number of electrons) to the cyanide ion. It too has two non-bonding pairs of electrons (see above). Why isn’t carbon monoxide a good nucleophile – the carbon atom and the oxygen atom both have one non-bonding pair of electrons they could use to approach a δ+ carbon atom? After all it is a good ligand and like cyanide ions when it acts as a Lewis base with transition metals again it is the non-bonding pair on the carbon atom, not the more electronegative oxygen atom, which forms the coordinate bond (as, for example, is shown in the image on the left for iron pentacarbonyl, Fe(CO)5).
I’ve asked a lot of questions here and given no answers. Shouldn’t we be training and encouraging students to ask these sorts of basic questions instead of just accepting what is on the syllabus without really understanding it?


Comments
To post comments you need to log in. If it is your first time you will need to subscribe.