Chemical misconceptions
Wednesday 28 JIB Docs (2) Teamary 2015
 We’ve all come across chemical misconceptions and part of our job as chemistry teachers it to try to combat them. For example, would you eat something that contains octadecadienoic acid, hexadecaenoic acid, E160a, E161c, phytosterols, 2,5-dimethyl-4-methoxy-2H-furan-3-one, E210 and methyl anthranilate amongst a host of other chemicals without first finding out whether it was likely to be carcinogenic? Well I would because all these chemicals plus many more are present in strawberries. – and I like strawberries!
We’ve all come across chemical misconceptions and part of our job as chemistry teachers it to try to combat them. For example, would you eat something that contains octadecadienoic acid, hexadecaenoic acid, E160a, E161c, phytosterols, 2,5-dimethyl-4-methoxy-2H-furan-3-one, E210 and methyl anthranilate amongst a host of other chemicals without first finding out whether it was likely to be carcinogenic? Well I would because all these chemicals plus many more are present in strawberries. – and I like strawberries!
Non-chemists seem to instinctively think that man-made, synthetic chemicals are inherently different to those that occur naturally. Are plants that are grown organically really able to distinguish between nitrate and phosphate ions in the soil that have come from natural fertilisers compared to those that have been added from artificial fertilisers? A helpful article published by Sense about Science titled ‘Making sense of chemical stories’ lists six major chemical misconceptions and provides good evidence to use to combat them.
The six misconceptions are:
1. You can lead a chemical-free life.
2. Man-made chemicals are inherently dangerous.
3. Synthetic chemicals are causing many cancers and diseases.
4. Our exposure to a cocktail of chemicals is a ticking time bomb.
5. It is beneficial to avoid man-made chemicals.
6. We are subjects in an unregulated, uncontrolled experiment.
It suggests that one of the reasons why people seem to be getting more and more worried about ‘chemicals’ is the huge advances made in analytical chemistry. It is now possible to determine the exact composition and amounts of extremely minute quantities of chemicals to be detected. Another reason is the proliferation of popular articles on science, which often tend to reinforce these misconceptions.
Last week the United States Environmental Protection Agency issued stricter guidelines on the use of long-chain perfluorinated chemicals, LCPFCs. One of these is perfluorooctanoic acid, PFOA.
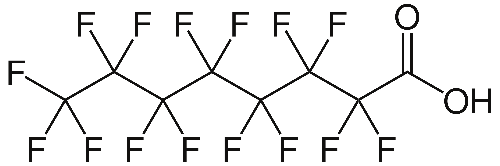
Perfluorooctanoic acid has been linked to an increase in tumours of the liver, pancreas and testicles of laboratory animals resulting in reduced fertility. PFOA is one of the chemicals used to make Teflon, the surface coating of non-stick saucepans and frying pans.
 Teflon itself is thought to be completely safe and free from PFOA because the mIB Docs (2) Teamfacturing process involves heating to high temperatures. This removes all the PFOA. Following the Environmental Protection Agency decision an article appeared the Guardian newspaper with the headline “Are my non-stick saucepans a health hazard?” The article lists the decision to limit PFOA, stresses its importance in the mIB Docs (2) Teamfacture of Teflon and its links to tumours. Only when you continue to read through the article and find that recent research on 26 non-stick cookware products tested under extreme conditions concluded that none of them released any noxious chemicals will you revise your first impression that non-stick pans are not safe to use.
Teflon itself is thought to be completely safe and free from PFOA because the mIB Docs (2) Teamfacturing process involves heating to high temperatures. This removes all the PFOA. Following the Environmental Protection Agency decision an article appeared the Guardian newspaper with the headline “Are my non-stick saucepans a health hazard?” The article lists the decision to limit PFOA, stresses its importance in the mIB Docs (2) Teamfacture of Teflon and its links to tumours. Only when you continue to read through the article and find that recent research on 26 non-stick cookware products tested under extreme conditions concluded that none of them released any noxious chemicals will you revise your first impression that non-stick pans are not safe to use.


Comments
To post comments you need to log in. If it is your first time you will need to subscribe.