A young chemist
Sunday 12 February 2012
I suppose in our heart of hearts IB Chemistry teachers know that they are unlikely to change the course of science by coming up with a revolutionary paradigm shift. It is not that we are lacking in intelligence and imagination – it is simply that most of us are just too old. By the time he was 23 Newton had discovered calculus, the law of gravitation and published Optics. Einstein was 26 when he published his three papers on special relativity, the photoelectric effect and the equivalence of matter and energy in 1905. Darwin was aged 22-27 when he came up with the theory of evolution on HMS Beagle. Perhaps we will just have to leave it to our young 17-19 year old IB Diploma students but unfortunately even they may now be too old by today’s standards.
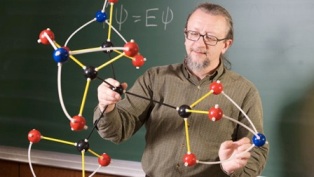 Ten year old Clara Lazen of Border Star Montessori School, Kansas City had been given a ball and stick molecular modelling kit by her school science teacher, Ken Boehr. The rules were that the black balls (carbon) must have four ‘sticks’ attached, the red balls (oxygen) two sticks and the blue balls (nitrogen) three sticks. She produced a model and asked her teacher whether it represented something that actually existed. Ken could not recognise it so sent a photograph of it to a friend, Dr Robert Zoellner (left) who is a lecturer in Chemistry at Humboldt State University in California. Zoellner confirmed that the compound she had modelled did not exist in nature and had not so far been synthesised synthetically but that it should be possible to make. He called the molecule tetranitratoxycarbon, C(CO3N)4 and predicts that it will have explosive properties. Zoellner has published an article on the possible isomers of tetranitratoxycarbon together with Boehr and Lazen as co-authors in Computational and Theoretical Chemistry.
Ten year old Clara Lazen of Border Star Montessori School, Kansas City had been given a ball and stick molecular modelling kit by her school science teacher, Ken Boehr. The rules were that the black balls (carbon) must have four ‘sticks’ attached, the red balls (oxygen) two sticks and the blue balls (nitrogen) three sticks. She produced a model and asked her teacher whether it represented something that actually existed. Ken could not recognise it so sent a photograph of it to a friend, Dr Robert Zoellner (left) who is a lecturer in Chemistry at Humboldt State University in California. Zoellner confirmed that the compound she had modelled did not exist in nature and had not so far been synthesised synthetically but that it should be possible to make. He called the molecule tetranitratoxycarbon, C(CO3N)4 and predicts that it will have explosive properties. Zoellner has published an article on the possible isomers of tetranitratoxycarbon together with Boehr and Lazen as co-authors in Computational and Theoretical Chemistry.
methane.jpg) There are some interesting Theory of Knowledge applications to this story. It is a good illustration of serendipity. Clara discovered the molecule ‘by accident’. How many teachers might have said that as they did not recognise it then it probably could not exist and so would have dismissed it? By not dismissing it Ken acted in a similar way to Fleming who came back to his lab to find mould growing on his unwashed petri dishes – rather than throwing them away Fleming followed up his observation and discovered penicillin – Ken Boehr followed up Clara’s model and Robert Zoellner confirmed that isomers of tetranitratoxycarbon should exist.
There are some interesting Theory of Knowledge applications to this story. It is a good illustration of serendipity. Clara discovered the molecule ‘by accident’. How many teachers might have said that as they did not recognise it then it probably could not exist and so would have dismissed it? By not dismissing it Ken acted in a similar way to Fleming who came back to his lab to find mould growing on his unwashed petri dishes – rather than throwing them away Fleming followed up his observation and discovered penicillin – Ken Boehr followed up Clara’s model and Robert Zoellner confirmed that isomers of tetranitratoxycarbon should exist.
Of course they do still have to be made!
A video of Clara's discovery ![]() from Fox News posted on YouTube.
from Fox News posted on YouTube.


Comments
To post comments you need to log in. If it is your first time you will need to subscribe.