DP Chemistry Questionbank

Topic 17: Equilibrium
Description
[N/A]Directly related questions
-
16N.1.hl.TZ0.25:
A mixture of 0.40 mol of CO (g) and 0.40 mol of H2 (g) was placed in a 1.00 dm3 vessel. The following equilibrium was established.
CO (g) + 2H2 (g)
CH3OH (g)
At equilibrium, the mixture contained 0.25 mol of CO (g). How many moles of H2 (g) and CH3OH (g) were present at equilibrium?
- 17M.1.hl.TZ1.23: The graph shows values of ΔG for a reaction at different temperatures. Which statement is...
-
17M.1.hl.TZ2.23:
Components X and Y are mixed together and allowed to reach equilibrium. The concentrations of X, Y, W and Z in the equilibrium mixture are 4, 1, 4 and 2 moldm−32 moldm−3 respectively.
X + 2Y ⇌⇌ 2W + Z
What is the value of the equilibrium constant, Kc?
A. 1818
B. 1212
C. 2
D. 8
-
17M.2.hl.TZ2.4d.i:
At a given time, the concentration of NO2(g) and N2O4(g) were 0.52 and 0.10 moldm−30.10 moldm−3 respectively.
Deduce, showing your reasoning, if the forward or the reverse reaction is favoured at this time.
-
17M.2.hl.TZ2.4d.ii:
Comment on the value of ΔG when the reaction quotient equals the equilibrium constant, Q = K.
- 20N.1.hl.TZ0.23: Which statement is correct for a spontaneous reaction?
-
17N.1.hl.TZ0.23:
At 700 ºC, the equilibrium constant, Kc, for the reaction is 1.075 × 108.
2H2 (g) + S2 (g) ⇌⇌ 2H2S (g)
Which relationship is always correct for the equilibrium at this temperature?
A. [H2S]2 < [H2]2 [S2]
B. [S2] = 2[H2S]
C. [H2S] < [S2]
D. [H2S]2 > [H2]2[S2]
- 17N.2.hl.TZ0.6a.ii: The following equilibrium concentrations in mol dm–3 were obtained at 761 K. Calculate the...
-
17N.2.hl.TZ0.6a.iii:
Determine the value of ΔGθ, in kJ, for the above reaction at 761 K using section 1 of the data booklet.
-
21M.1.hl.TZ1.23:
1.0 mol each of sulfur dioxide, oxygen, and sulfur trioxide are in equilibrium.
2SO2 (g)+O2 (g)⇌2SO3 (g)2SO2(g)+O2(g)⇌2SO3(g)
Which change in the molar ratio of reactants will cause the greatest increase in the amount of sulfur trioxide?
Assume volume and temperature of the reaction mixture remain constant.
-
21M.2.hl.TZ1.4e(i):
The equilibrium constant, Kc, has a value of 1.01 at 298 K.
Calculate ΔG⦵, in kJ mol–1, for this reaction. Use sections 1 and 2 of the data booklet.
-
21M.2.hl.TZ2.7c:
SO2 (g), O2 (g) and SO3 (g) are mixed and allowed to reach equilibrium at 600 °C.
Determine the value of Kc at 600 °C.
-
18M.2.hl.TZ1.1d.iii:
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
-
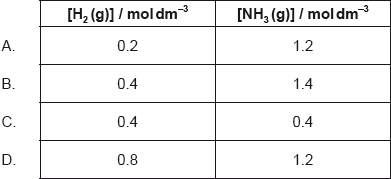
18M.1.hl.TZ1.23:
1.0 mol of N2(g), 1.0 mol of H2(g) and 1.0 mol of NH3(g) are placed in a 1.0 dm3 sealed flask and left to reach equilibrium. At equilibrium the concentration of N2(g) is 0.8 mol dm−3.
N2(g) + 3H2(g) ⇌⇌ 2NH3(g)
What are the equilibrium concentration of H2(g) and NH3(g) in mol dm−3?
-
18M.2.hl.TZ2.6c.ii:
A two-step mechanism is proposed for the formation of NO2(g) from NO(g) that involves an exothermic equilibrium process.
First step: 2NO(g) ⇌⇌ N2O2(g) fast
Second step: N2O2(g) + O2 (g) → 2NO2(g) slow
Deduce the rate expression for the mechanism.
-
21N.1.hl.TZ0.23:
The graph shows Gibbs free energy of a mixture of N2O4 (g) and NO2 (g) in different proportions.
N2O4 (g) ⇌⇌ 2NO2 (g)
Which point shows the system at equilibrium?
-
21N.2.hl.TZ0.3c(iv):
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
- 18N.1.hl.TZ0.23: Which combination describes the system at equilibrium?
- 18N.1.hl.TZ0.30: Which is correct for a redox reaction where the standard electrode potential is negative? ΔGΘ =...
-
18N.2.hl.TZ0.5c:
Calculate the standard Gibbs free energy change, ΔGΘ, in kJ, for this reaction at 1000 K. Use sections 1 and 2 of the data booklet.
- 22M.2.hl.TZ1.3c(i): State, giving a reason, whether the reaction is spontaneous or not at 298 K.
-
22M.2.hl.TZ1.3c(ii):
Calculate the value of the equilibrium constant, K, at 298 K. Use sections 1 and 2 of the data booklet.
-
22M.2.hl.TZ2.4d(iv):
Calculate the equilibrium constant, Kc, for this reaction at 298 K. Use your answer to (d)(iii) and sections 1 and 2 of the data booklet.
(If you did not obtain an answer to (d)(iii) use a value of 2.0 kJ mol−1, although this is not the correct answer).
-
19M.2.hl.TZ1.6f(iii):
Calculate a value for the equilibrium constant, Kc, at 298 K, giving your answer to two significant figures. Use your answer to (f)(ii) and section 1 of the data booklet.
(If you did not obtain an answer to (f)(ii), use −140 kJ mol−1, but this is not the correct value.)
-
19M.2.hl.TZ2.6b:
Phenylethene is manufactured from benzene and ethene in a two-stage process. The overall reaction can be represented as follows with ΔGθ = +10.0 kJ mol−1 at 298 K.
Calculate the equilibrium constant for the overall conversion at 298 K, using section 1 of the data booklet.
- 19M.1.hl.TZ1.23: Which is correct for a reaction with a positive change in Gibbs free energy, ΔGθ? A. The...
- 19M.1.hl.TZ2.23: Iodine and bromine gases were mixed and allowed to reach equilibrium. What is the value of the...
- 19N.1.hl.TZ0.24: Which corresponds to a system at equilibrium?
Sub sections and their related questions
17.1 The equilibrium law
-
16N.1.hl.TZ0.25:
A mixture of 0.40 mol of CO (g) and 0.40 mol of H2 (g) was placed in a 1.00 dm3 vessel. The following equilibrium was established.
CO (g) + 2H2 (g)
CH3OH (g)
At equilibrium, the mixture contained 0.25 mol of CO (g). How many moles of H2 (g) and CH3OH (g) were present at equilibrium?
- 17M.1.hl.TZ1.23: The graph shows values of ΔG for a reaction at different temperatures. Which statement is...
-
17M.1.hl.TZ2.23:
Components X and Y are mixed together and allowed to reach equilibrium. The concentrations of X, Y, W and Z in the equilibrium mixture are 4, 1, 4 and 2 moldm−32 moldm−3 respectively.
X + 2Y ⇌⇌ 2W + Z
What is the value of the equilibrium constant, Kc?
A. 1818
B. 1212
C. 2
D. 8
-
17M.2.hl.TZ2.4d.i:
At a given time, the concentration of NO2(g) and N2O4(g) were 0.52 and 0.10 moldm−30.10 moldm−3 respectively.
Deduce, showing your reasoning, if the forward or the reverse reaction is favoured at this time.
-
17M.2.hl.TZ2.4d.ii:
Comment on the value of ΔG when the reaction quotient equals the equilibrium constant, Q = K.
-
17N.1.hl.TZ0.23:
At 700 ºC, the equilibrium constant, Kc, for the reaction is 1.075 × 108.
2H2 (g) + S2 (g) ⇌⇌ 2H2S (g)
Which relationship is always correct for the equilibrium at this temperature?
A. [H2S]2 < [H2]2 [S2]
B. [S2] = 2[H2S]
C. [H2S] < [S2]
D. [H2S]2 > [H2]2[S2]
- 17N.2.hl.TZ0.6a.ii: The following equilibrium concentrations in mol dm–3 were obtained at 761 K. Calculate the...
-
17N.2.hl.TZ0.6a.iii:
Determine the value of ΔGθ, in kJ, for the above reaction at 761 K using section 1 of the data booklet.
-
18M.1.hl.TZ1.23:
1.0 mol of N2(g), 1.0 mol of H2(g) and 1.0 mol of NH3(g) are placed in a 1.0 dm3 sealed flask and left to reach equilibrium. At equilibrium the concentration of N2(g) is 0.8 mol dm−3.
N2(g) + 3H2(g) ⇌⇌ 2NH3(g)
What are the equilibrium concentration of H2(g) and NH3(g) in mol dm−3?

-
18M.2.hl.TZ1.1d.iii:
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
-
18M.2.hl.TZ2.6c.ii:
A two-step mechanism is proposed for the formation of NO2(g) from NO(g) that involves an exothermic equilibrium process.
First step: 2NO(g) ⇌⇌ N2O2(g) fast
Second step: N2O2(g) + O2 (g) → 2NO2(g) slow
Deduce the rate expression for the mechanism.
- 18N.1.hl.TZ0.23: Which combination describes the system at equilibrium?
- 18N.1.hl.TZ0.30: Which is correct for a redox reaction where the standard electrode potential is negative? ΔGΘ =...
-
18N.2.hl.TZ0.5c:
Calculate the standard Gibbs free energy change, ΔGΘ, in kJ, for this reaction at 1000 K. Use sections 1 and 2 of the data booklet.
-
19M.2.hl.TZ1.6f(iii):
Calculate a value for the equilibrium constant, Kc, at 298 K, giving your answer to two significant figures. Use your answer to (f)(ii) and section 1 of the data booklet.
(If you did not obtain an answer to (f)(ii), use −140 kJ mol−1, but this is not the correct value.)
-
19M.2.hl.TZ2.6b:
Phenylethene is manufactured from benzene and ethene in a two-stage process. The overall reaction can be represented as follows with ΔGθ = +10.0 kJ mol−1 at 298 K.
Calculate the equilibrium constant for the overall conversion at 298 K, using section 1 of the data booklet.
- 19M.1.hl.TZ1.23: Which is correct for a reaction with a positive change in Gibbs free energy, ΔGθ? A. The...
- 19M.1.hl.TZ2.23: Iodine and bromine gases were mixed and allowed to reach equilibrium. What is the value of the...
- 19N.1.hl.TZ0.24: Which corresponds to a system at equilibrium?
- 20N.1.hl.TZ0.23: Which statement is correct for a spontaneous reaction?
-
21M.1.hl.TZ1.23:
1.0 mol each of sulfur dioxide, oxygen, and sulfur trioxide are in equilibrium.
2SO2 (g)+O2 (g)⇌2SO3 (g)2SO2(g)+O2(g)⇌2SO3(g)
Which change in the molar ratio of reactants will cause the greatest increase in the amount of sulfur trioxide?
Assume volume and temperature of the reaction mixture remain constant.
-
21M.2.hl.TZ1.4e(i):
The equilibrium constant, Kc, has a value of 1.01 at 298 K.
Calculate ΔG⦵, in kJ mol–1, for this reaction. Use sections 1 and 2 of the data booklet.
-
21M.2.hl.TZ2.7c:
SO2 (g), O2 (g) and SO3 (g) are mixed and allowed to reach equilibrium at 600 °C.
Determine the value of Kc at 600 °C.
-
21N.1.hl.TZ0.23:
The graph shows Gibbs free energy of a mixture of N2O4 (g) and NO2 (g) in different proportions.
N2O4 (g) ⇌⇌ 2NO2 (g)
Which point shows the system at equilibrium?
-
21N.2.hl.TZ0.3c(iv):
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
- 22M.2.hl.TZ1.3c(i): State, giving a reason, whether the reaction is spontaneous or not at 298 K.
-
22M.2.hl.TZ1.3c(ii):
Calculate the value of the equilibrium constant, K, at 298 K. Use sections 1 and 2 of the data booklet.
-
22M.2.hl.TZ2.4d(iv):
Calculate the equilibrium constant, Kc, for this reaction at 298 K. Use your answer to (d)(iii) and sections 1 and 2 of the data booklet.
(If you did not obtain an answer to (d)(iii) use a value of 2.0 kJ mol−1, although this is not the correct answer).
