
SL Paper 1
Which change of state is exothermic?
A. CO2(s) → CO2(g)
B. H2O(l) → H2O(g)
C. NH3(g) → NH3(l)
D. Fe(s) → Fe(l)
Which expression gives the mass, in g, of ethanol required to produce 683.5 kJ of heat upon complete combustion?
(Mr for ethanol = 46.0, )
A.
B.
C.
D.
Which statement is correct for this reaction?
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g) ΔH = −26.6 kJ
A. 13.3 kJ are released for every mole of Fe produced.
B. 26.6 kJ are absorbed for every mole of Fe produced.
C. 53.2 kJ are released for every mole of Fe produced.
D. 26.6 kJ are released for every mole of Fe produced.
What is the enthalpy change, in J, when 5 g of water is heated from 10°C to 18°C?
Specific heat capacity of water: 4.18 kJ kg−1 K−1
A. 5 × 4.18 × 8
B. 5 × 10−3 × 4.18 × 8
C. 5 × 4.18 × (273 + 8)
D. 5 × 10−3 × 4.18 × (273 + 8)
What can be deduced from this reaction profile?
A. The reactants are less stable than the products and the reaction is exothermic.
B. The reactants are less stable than the products and the reaction is endothermic.
C. The reactants are more stable than the products and the reaction is exothermic.
D. The reactants are more stable than the products and the reaction is endothermic.
Hydrazine reacts with oxygen.
N2H4(l) + O2(g) → N2(g) + 2H2O(l) ΔHθ = -623 kJ
What is the standard enthalpy of formation of N2H4(l) in kJ? The standard enthalpy of formation of H2O(l) is -286 kJ.
A. -623 - 286
B. -623 + 572
C. -572 + 623
D. -286 + 623
What is correct about energy changes during bond breaking and bond formation?
Which combination will give you the enthalpy change for the hydrogenation of ethene to ethane, ?
A.
B.
C.
D.
What can be deduced from the facts that ozone absorbs UV radiation in the region of 340 nm and molecular oxygen in the region of 242 nm?
A. The bond between atoms in molecular oxygen is a double bond.
B. The bonds in ozone are delocalized.
C. The bonds between atoms in ozone are stronger than those in molecular oxygen.
D. The bonds between atoms in molecular oxygen need more energy to break.
Consider the following reaction:
N2 (g) + 3H2 (g) 2NH3 (g)
Which calculation gives ΔHΘ, in kJ, for the forward reaction?
A. 2z − y − 3x
B. y + 3x − 2z
C. y + 3x − 6z
D. 6z − y − 3x
5.35g of solid ammonium chloride, NH4Cl(s), was added to water to form 25.0g of solution. The maximum decrease in temperature was 14 K. What is the enthalpy change, in kJmol-1, for this reaction? (Molar mass of NH4Cl = 53.5gmol-1; the specific heat capacity of the solution is 4.18 Jg-1K-1)
A.
B.
C.
D.
Which equation represents the standard enthalpy of formation of lithium oxide?
A. 4Li (s) + O2 (g) → 2Li2O (s)
B. 2Li (s) + O2 (g) → Li2O (s)
C. Li (s) + O2 (g) → Li2O (s)
D. Li (g) + O2 (g) → Li2O (g)
The enthalpy of combustion of a fuel was determined using the calorimeter shown. The final result was lower than the literature value.
Which factors could have contributed to this error?
I. Not all heat from the combustion was transferred to the calorimeter.
II. Incomplete combustion occurred.
III. The temperature probe touched the bottom of the calorimeter.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Questions 13 and 14 are about an experiment to measure the enthalpy of combustion, ΔHc, of ethanol, using the apparatus and setup shown.
Which quantity is likely to be the most inaccurate due to the sources of error in this experiment?
A. Mass of ethanol burnt
B. Molecular mass of ethanol
C. Mass of water
D. Temperature change
Methane undergoes incomplete combustion.
2CH4 (g) + 3O2 (g) → 2CO (g) + 4H2O (g)
What is the enthalpy change, in kJ, using the bond enthalpy data given below?
A. [2(1077) + 4(463)] − [2(414) + 3(498)]
B. [2(414) + 3(498)] − [2(1077) + 4(463)]
C. [8(414) + 3(498)] − [2(1077) + 8(463)]
D. [2(1077) + 8(463)] − [8(414) + 3(498)]
What is the enthalpy change of the following reaction?
CH2CHCH2CH3 + HBr → CH3CHBrCH2CH3
A. –119.6 kJ
B. +119.6 kJ
C. –119.8 kJ
D. +119.8 kJ
What is the enthalpy change of reaction for the following equation?
A. x + y + z
B. −x − y + z
C. x − y − z
D. x − y + z
Consider the following equations.
2Al (s) + O2 (g) → Al2O3 (s) ΔHƟ = −1670 kJ
Mn (s) + O2 (g) → MnO2 (s) ΔHƟ = −520 kJ
What is the standard enthalpy change, in kJ, of the reaction below?
4Al (s) + 3MnO2 (s) → 2Al2O3 (s) + 3Mn (s)
A. −1670 + 520
B. (−1670) + 3(520)
C. 2(−1670) + 3(−520)
D. 2(−1670) + 3(520)
When equal masses of X and Y absorb the same amount of energy, their temperatures rise by 5 °C and 10 °C respectively. Which is correct?
A. The specific heat capacity of X is twice that of Y.
B. The specific heat capacity of X is half that of Y.
C. The specific heat capacity of X is one fifth that of Y.
D. The specific heat capacity of X is the same as Y.
Consider the following reactions:
Fe2O3 (s) + CO (g) → 2FeO (s) + CO2 (g) ΔHΘ = −3 kJ
Fe (s) + CO2 (g) → FeO (s) + CO (g) ΔHΘ = +11 kJ
What is the ΔHΘ value, in kJ, for the following reaction?
Fe2O3 (s) + 3CO (g) → 2Fe (s) + 3CO2 (g)
A. −25
B. −14
C. +8
D. +19
Which statement is correct about identical pieces of magnesium added to two solutions, X and Y, containing hydrochloric acid at the same temperature?
A. Solution X will reach a higher maximum temperature.
B. Solution Y will reach a higher maximum temperature.
C. Solutions X and Y will have the same temperature rise.
D. It is not possible to predict whether X or Y will have the higher maximum temperature because we cannot identify the limiting reactant.
Which describes the reaction shown in the potential energy profile?
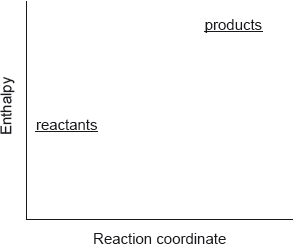
A. The reaction is endothermic and the products have greater enthalpy than the reactants.
B. The reaction is endothermic and the reactants have greater enthalpy than the products.
C. The reaction is exothermic and the products have greater enthalpy than the reactants.
D. The reaction is exothermic and the reactants have greater enthalpy than the products.
What is the heat change, in kJ, when 100.0 g of aluminium is heated from 19.0 °C to 32.0 °C?
Specific heat capacity of aluminium: 0.90 J g−1 K−1
A.
B.
C.
D.
In which order does the oxygen–oxygen bond enthalpy increase?
A. H2O2 < O2 < O3
B. H2O2 < O3 < O2
C. O2 < O3 < H2O2
D. O3 < H2O2 < O2
A student obtained the following data to calculate , using .
What is the percentage uncertainty in the calculated value of ?
A.
B.
C.
D.
Which combustion reaction releases the least energy per mole of C3H8?
Approximate bond enthalpy / kJ mol−1
O=O 500
C=O 800
C≡O 1000
A. C3H8 (g) + 5O2 (g) → 3CO2 (g) + 4H2O (g)
B. C3H8 (g) + O2 (g) → 2CO2 (g) + CO (g) + 4H2O (g)
C. C3H8 (g) + 4O2 (g) → CO2 (g) + 2CO (g) + 4H2O (g)
D. C3H8 (g) + O2 (g) → 3CO (g) + 4H2O (g)
Chemistry: Atoms First 2e, https://openstax.org/books/chemistry-atoms-first-2e/pages/9-4-strengths-of-ionic-andcovalent-bonds © 1999–2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License.
(CC BY 4.0) https://creativecommons.org/licenses/ by/4.0/.
Which statement describes an endothermic reaction?
A. The bonds broken are stronger than the bonds formed.
B. The enthalpy of the reactants is higher than the enthalpy of the products.
C. The temperature of the surroundings increases.
D. The products are more stable than the reactants.
The enthalpy changes for two reactions are given.
Br2 (l) + F2 (g) → 2BrF (g) ΔH = x kJ
Br2 (l) + 3F2 (g) → 2BrF3 (g) ΔH = y kJ
What is the enthalpy change for the following reaction?
BrF (g) + F2 (g) → BrF3 (g)
A. x – y
B. –x + y
C. (–x + y)
D. (x – y)
Which equation shows the enthalpy of formation, , of ethanol?
A.
B.
C.
D.
What is the enthalpy change of the reaction?
C6H14 (l) → C2H4 (g) + C4H10 (g)
A. + 1411 + 2878 + 4163
B. + 1411 − 2878 − 4163
C. + 1411 + 2878 − 4163
D. − 1411 − 2878 + 4163
When sodium carbonate powder is added to ethanoic acid, the beaker becomes cooler.
Possible enthalpy diagrams are shown.
Which correctly describes the reaction?
The C=N bond has a bond length of 130 pm and an average bond enthalpy of 615kJmol-1. Which values would be most likely for the C-N bond?
Which is correct when Ba(OH)2 reacts with NH4Cl?
Ba(OH)2 (s) + 2NH4Cl (s) → BaCl2 (aq) + 2NH3 (g) + 2H2O (l) ΔHΘ = +164 kJ mol−1
In which reaction do the reactants have a lower potential energy than the products?
A. CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
B. HBr(g) → H(g) + Br(g)
C. Na+(g) + Cl-(g) → NaCl(s)
D. NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
What is the enthalpy change, in kJ, of the following reaction?
3H2 (g) + N2 (g) 2NH3 (g)
A. (6 × 391) − [(3 × 436) + 945]
B. (3 × 391) − (436 + 945)
C. −[(3 × 436) + 945] + (3 × 391)
D. −(6 × 391) + [(3 × 436) + 945]
Which is the enthalpy change of reaction, ΔH?
Which equation represents the N–H bond enthalpy in NH3?
A. NH3 (g) → N (g) + 3H (g)
B. NH3 (g) → N (g) + H (g)
C. NH3 (g) → N2 (g) + H2 (g)
D. NH3 (g) → •NH2 (g) + •H (g)
Two 100 cm3 aqueous solutions, one containing 0.010 mol NaOH and the other 0.010 mol HCl, are at the same temperature.
When the two solutions are mixed the temperature rises by y °C.
Assume the density of the final solution is 1.00 g cm−3.
Specific heat capacity of water = 4.18 J g−1 K−1
What is the enthalpy change of neutralization in kJ mol−1?
A.
B.
C.
D.
The energy from burning 0.250 g of ethanol causes the temperature of 150 cm3 of water to rise by 10.5 °C. What is the enthalpy of combustion of ethanol, in kJ mol–1?
Specific heat capacity of water: 4.18 J g–1 K–1.
A.
B.
C.
D.
Which describes an exothermic reaction?
What is the enthalpy of combustion of butane in kJ mol−1?
2C4H10(g) + 13O2(g) → 8CO2(g) + 10H2O(l)
A. 4x + 5y − z
B. 4x + 5y + z
C. 8x + 10y − 2z
D. 8x + 5y + 2z
Why is the value of the enthalpy change of this reaction calculated from bond enthalpy data less accurate than that calculated from standard enthalpies of formation?
2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(g)
A. All the reactants and products are gases.
B. Bond enthalpy data are average values for many compounds.
C. Elements do not have standard enthalpy of formation.
D. Standard enthalpies of formation are per mole.
What is the enthalpy change of combustion of urea, (NH2)2CO, in kJ mol−1?
2(NH2)2CO(s) + 3O2(g) → 2CO2(g) + 2N2(g) + 4H2O(l)
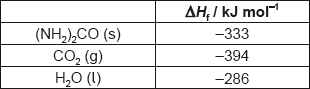
A. 2 × (−333) −2 × (−394) −4 × (−286)
B. [2 × (−394) + 4 × (−286) −2 × (−333)]
C. 2 × (−394) + 4 × (−286) −2 × (−333)
D. [2 × (−333) −2 × (−394) −4 × (−286)]
Which is correct for the reaction?
2Al (s) + 6HCl (aq) → 2AlCl3 (aq) + 3H2 (g) ΔH = −1049 kJ
A. Reactants are less stable than products and the reaction is endothermic.
B. Reactants are more stable than products and the reaction is endothermic.
C. Reactants are more stable than products and the reaction is exothermic.
D. Reactants are less stable than products and the reaction is exothermic.
What is the bond enthalpy, in , in the molecule?
A.
B.
C.
D.
Which expression gives the enthalpy change, ΔH, for the thermal decomposition of calcium carbonate?
A. ΔH = ΔH1 − ΔH2
B. ΔH = 2ΔH1 − ΔH2
C. ΔH = ΔH1 − 2ΔH2
D. ΔH = ΔH1 + ΔH2
What is the enthalpy change of the reaction, in kJ?
2C (graphite) + O2 (g) → 2CO (g)
A. −394 − 283
B. 2(−394) + 2(−283)
C. −394 + 283
D. 2(−394) + 2(283)
Which combination of ΔH1, ΔH2, and ΔH3 would give the enthalpy of the reaction?
CS2 (l) + 3O2 (g) → CO2 (g) + 2SO2 (g)
ΔH1 C (s) + O2 (g) → CO2 (g)
ΔH2 S (s) + O2 (g) → SO2 (g)
ΔH3 C (s) + 2S (s) → CS2 (l)
A. ΔH = ΔH1 + ΔH2 + ΔH3
B. ΔH = ΔH1 + ΔH2 − ΔH3
C. ΔH = ΔH1 + 2(ΔH2) + ΔH3
D. ΔH = ΔH1 + 2(ΔH2) − ΔH3
Which statement is correct?
A. In an exothermic reaction, the products have more energy than the reactants.
B. In an exothermic reversible reaction, the activation energy of the forward reaction is greater than that of the reverse reaction.
C. In an endothermic reaction, the products are more stable than the reactants.
D. In an endothermic reversible reaction, the activation energy of the forward reaction is greater than that of the reverse reaction.
What is the correct interpretation of the following potential energy profile?
A. Endothermic reaction; products more stable than reactants.
B. Exothermic reaction; products more stable than reactants.
C. Endothermic reaction; products less stable than reactants.
D. Exothermic reaction; products less stable than reactants.
Questions 13 and 14 are about an experiment to measure the enthalpy of combustion, ΔHc, of ethanol, using the apparatus and setup shown.
What is the enthalpy of combustion, ΔHc, of ethanol in kJ mol−1?
Maximum temperature of water: 30.0°C
Initial temperature of water: 20.0°C
Mass of water in beaker: 100.0 g
Loss in mass of ethanol: 0.230 g
Mr (ethanol): 46.08
Specific heat capacity of water: 4.18 J g−1 K−1
q = mcΔT
A.
B.
C.
D.
The enthalpy of combustion of ethanol is determined by heating a known mass of tap water in a glass beaker with a flame of burning ethanol.
Which will lead to the greatest error in the final result?
A. Assuming the density of tap water is 1.0 g cm−3
B. Assuming all the energy from the combustion will heat the water
C. Assuming the specific heat capacity of the tap water is 4.18 J g−1 K−1
D. Assuming the specific heat capacity of the beaker is negligible