
HL Paper 2
Cobalt forms the transition metal complex [Co(NH3)4 (H2O)Cl]Br.
Trends in physical and chemical properties are useful to chemists.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group whereas the melting points of the group 17 elements (F → I) increase down the group.
State the shape of the complex ion.
Deduce the charge on the complex ion and the oxidation state of cobalt.
Describe, in terms of acid-base theories, the type of reaction that takes place between the cobalt ion and water to form the complex ion.
An equation for the combustion of propane is given below.
C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g)
Determine the standard enthalpy change, , for this reaction, using section 11 of the data booklet.
Calculate the standard enthalpy change, , for this reaction using section 12 of the data booklet.
Predict, giving a reason, whether the entropy change, , for this reaction is negative or positive.
Calculate for the reaction in , using section 12 of the data booklet.
The standard molar entropy for oxygen gas is .
Calculate the standard Gibbs free energy change, , in , for the reaction at 5 °C, using your answers to (b) and (d). Use section 1 of the data booklet.
(If you did not obtain an answer to (b) or (d) use values of and respectively, although these are not the correct answers.)
Ammonia is produced by the Haber–Bosch process which involves the equilibrium:
N2 (g) + 3 H2 (g) 2 NH3 (g)
The percentage of ammonia at equilibrium under various conditions is shown:
[The Haber Bosch Process [graph] Available at: https://commons.wikimedia.org/wiki/File:Ammonia_yield.png
[Accessed: 16/07/2022].]
One factor affecting the position of equilibrium is the enthalpy change of the reaction.
The standard free energy change, ΔG⦵, for the Haber–Bosch process is –33.0 kJ at 298 K.
Deduce the expression for the equilibrium constant, Kc, for this equation.
State how the use of a catalyst affects the position of the equilibrium.
With reference to the reaction quotient, Q, explain why the percentage yield increases as the pressure is increased at constant temperature.
Determine the enthalpy change, ΔH, for the Haber–Bosch process, in kJ. Use Section 11 of the data booklet.
Outline why the value obtained in (b)(i) might differ from a value calculated using ΔHf data.
Demonstrate that your answer to (b)(i) is consistent with the effect of an increase in temperature on the percentage yield, as shown in the graph.
State, giving a reason, whether the reaction is spontaneous or not at 298 K.
Calculate the value of the equilibrium constant, K, at 298 K. Use sections 1 and 2 of the data booklet.
Calculate the entropy change for the Haber–Bosch process, in J mol–1 K–1 at 298 K. Use your answer to (b)(i) and section 1 of the data booklet.
Outline, with reference to the reaction equation, why this sign for the entropy change is expected.
Oxygen exists as two allotropes, diatomic oxygen, O2, and ozone, O3.
Draw a Lewis (electron dot) structure for ozone.
Discuss the relative length of the two O−O bonds in ozone.
Explain why there are frequencies of UV light that will dissociate O3 but not O2.
Explain, using equations, how the presence of results in a chain reaction that decreases the concentration of ozone in the stratosphere.
Carbon forms many compounds.
C60 and diamond are allotropes of carbon.
Chlorine reacts with methane.
CH4 (g) + Cl2 (g) → CH3Cl (g) + HCl (g)
Outline two differences between the bonding of carbon atoms in C60 and diamond.
Explain why C60 and diamond sublime at different temperatures and pressures.
State two features showing that propane and butane are members of the same homologous series.
Describe a test and the expected result to indicate the presence of carbon–carbon double bonds.
Draw the full structural formula of (Z)-but-2-ene.
Write the equation for the reaction between but-2-ene and hydrogen bromide.
State the type of reaction.
Suggest two differences in the 1H NMR of but-2-ene and the organic product from (d)(ii).
Predict, giving a reason, the major product of reaction between but-1-ene and steam.
Explain the mechanism of the reaction between 1-bromopropane, CH3CH2CH2Br, and aqueous sodium hydroxide, NaOH (aq), using curly arrows to represent the movement of electron pairs.
Deduce the splitting pattern in the 1H NMR spectrum for 1-bromopropane.
Calculate the enthalpy change of the reaction, ΔH, using section 11 of the data booklet.
Draw and label an enthalpy level diagram for this reaction.
Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Now consider the second stage of the reaction.
CO (g) + 2H2 (g) CH3OH (l) ΔH⦵ = –129 kJ
Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Methanol is usually manufactured from methane in a two-stage process.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
CO (g) + 2H2 (g) CH3OH (l)
Consider the first stage of the reaction.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
The diagram shows the Maxwell-Boltzmann curve for the uncatalyzed reaction.
Draw a distribution curve at a lower temperature (T2) and show on the diagram how the addition of a catalyst enables the reaction to take place more rapidly than at T1.
The hydrogen peroxide could cause further oxidation of the methanol. Suggest a possible oxidation product.
Determine the overall equation for the production of methanol.
8.00 g of methane is completely converted to methanol. Calculate, to three significant figures, the final volume of hydrogen at STP, in dm3. Use sections 2 and 6 of the data booklet.
Determine the enthalpy change, ΔH, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
State one reason why you would expect the value of ΔH calculated from the values, given in section 12 of data booklet, to differ from your answer to (d)(i).
State the expression for Kc for this stage of the reaction.
State and explain the effect of increasing temperature on the value of Kc.
The equilibrium constant, Kc, has a value of 1.01 at 298 K.
Calculate ΔG⦵, in kJ mol–1, for this reaction. Use sections 1 and 2 of the data booklet.
Calculate a value for the entropy change, ΔS⦵, in J K–1 mol–1 at 298 K. Use your answers to (e)(i) and section 1 of the data booklet.
If you did not get answers to (e)(i) use –1 kJ, but this is not the correct answer.
Justify the sign of ΔS with reference to the equation.
Predict, giving a reason, how a change in temperature from 298 K to 273 K would affect the spontaneity of the reaction.
Ethyne, C2H2, reacts with oxygen in welding torches.
Ethyne reacts with steam.
C2H2 (g) + H2O (g) → C2H4O (g)
Two possible products are:
Product B, CH3CHO, can also be synthesized from ethanol.
Write an equation for the complete combustion of ethyne.
Deduce the Lewis (electron dot) structure of ethyne.
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
State the name of product B, applying IUPAC rules.
Determine the enthalpy change for the reaction, in kJ, to produce A using section 11 of the data booklet.
The enthalpy change for the reaction to produce B is −213 kJ.
Predict, giving a reason, which product is the most stable.
The IR spectrum and low resolution 1H NMR spectrum of the actual product formed are shown.
Deduce whether the product is A or B, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:
One piece of evidence from IR:
One piece of evidence from 1H NMR:
Deduce the splitting pattern you would expect for the signals in a high resolution 1H NMR spectrum.
2.3 ppm:
9.8 ppm:
Suggest the reagents and conditions required to ensure a good yield of product B.
Reagents:
Conditions:
Deduce the average oxidation state of carbon in product B.
Explain why product B is water soluble.
This question is about ethene, C2H4, and ethyne, C2H2.
Ethyne, like ethene, undergoes hydrogenation to form ethane. State the conditions required.
Outline the formation of polyethene from ethene by drawing three repeating units of the polymer.
Ethyne reacts with chlorine in a similar way to ethene. Formulate equations for the following reactions.
Under certain conditions, ethyne can be converted to benzene.
Determine the standard enthalpy change, ΔHΘ, for the reaction stated, using section 11 of the data booklet.
3C2H2(g) → C6H6(g)
Determine the standard enthalpy change, ΔHΘ, for the following similar reaction, using ΔHf values in section 12 of the data booklet.
3C2H2(g) → C6H6(l)
Explain, giving two reasons, the difference in the values for (c)(i) and (ii). If you did not obtain answers, use −475 kJ for (i) and −600 kJ for (ii).
Calculate the standard entropy change, ΔSΘ, in J K−1, for the reaction in (ii) using section 12 of the data booklet.
Determine, showing your working, the spontaneity of the reaction in (ii) at 25 °C.
One possible Lewis structure for benzene is shown.
State one piece of physical evidence that this structure is incorrect.
The photochemical chlorination of methane can occur at low temperature.
The overall equation for monochlorination of methane is:
CH4(g) + Cl2(g) → CH3Cl(g) + HCl(g)
Calculate the standard enthalpy change for the reaction, ΔH θ, using section 12 of the data booklet.
Soluble acids and bases ionize in water.
A solution containing 0.510 g of an unknown monoprotic acid, HA, was titrated with 0.100 mol dm–3 NaOH(aq). 25.0 cm3 was required to reach the equivalence point.
The following curve was obtained using a pH probe.
State, giving a reason, the strength of the acid.
State a technique other than a pH titration that can be used to detect the equivalence point.
Deduce the pKa for this acid.
The pKa of an anthocyanin is 4.35. Determine the pH of a 1.60 × 10–3 mol dm–3 solution to two decimal places.
Limestone can be converted into a variety of useful commercial products through the lime cycle. Limestone contains high percentages of calcium carbonate, CaCO3.
Thermodynamic data for the decomposition of calcium carbonate is given.
The second step of the lime cycle produces calcium hydroxide, Ca(OH)2.
Calcium hydroxide reacts with carbon dioxide to reform calcium carbonate.
Ca(OH)2 (aq) + CO2 (g) → CaCO3 (s) + H2O (l)
Calcium carbonate is heated to produce calcium oxide, CaO.
CaCO3 (s) → CaO (s) + CO2 (g)
Calculate the volume of carbon dioxide produced at STP when 555 g of calcium carbonate decomposes. Use sections 2 and 6 of the data booklet.
Calculate the enthalpy change of reaction, ΔH, in kJ, for the decomposition of calcium carbonate.
Calculate the change in entropy, ΔS, in J K−1, for the decomposition of calcium carbonate.
Determine the temperature, in K, at which the decomposition of calcium carbonate becomes spontaneous, using b(i), b(ii) and section 1 of the data booklet.
(If you do not have answers for b(i) and b(ii), use ΔH = 190 kJ and ΔS = 180 J K−1, but these are not the correct answers.)
Sketch an energy profile for the decomposition of calcium carbonate based on your answer to b(i), labelling the axes and activation energy, Ea.
State how adding a catalyst to the reaction would impact the enthalpy change of reaction, ΔH, and the activation energy, Ea.
Write the equation for the reaction of Ca(OH)2 (aq) with hydrochloric acid, HCl (aq).
Determine the volume, in dm3, of 0.015 mol dm−3 calcium hydroxide solution needed to neutralize 35.0 cm3 of 0.025 mol dm−3 HCl (aq).
Saturated calcium hydroxide solution is used to test for carbon dioxide. Calculate the pH of a 2.33 × 10−2 mol dm−3 solution of calcium hydroxide, a strong base.
Determine the mass, in g, of CaCO3 (s) produced by reacting 2.41 dm3 of 2.33 × 10−2 mol dm−3 of Ca(OH)2 (aq) with 0.750 dm3 of CO2 (g) at STP.
2.85 g of CaCO3 was collected in the experiment in d(i). Calculate the percentage yield of CaCO3.
(If you did not obtain an answer to d(i), use 4.00 g, but this is not the correct value.)
Outline how one calcium compound in the lime cycle can reduce a problem caused by acid deposition.
Enthalpy changes depend on the number and type of bonds broken and formed.
Enthalpy changes depend on the number and type of bonds broken and formed.
The table lists the standard enthalpies of formation, , for some of the species in the reaction above.
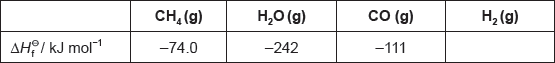
Hydrogen gas can be formed industrially by the reaction of natural gas with steam.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Determine the enthalpy change, ΔH, for the reaction, in kJ, using section 11 of the data booklet.
Bond enthalpy for C≡O: 1077 kJ mol−1
Outline why no value is listed for H2(g).
Determine the value of ΔHΘ, in kJ, for the reaction using the values in the table.
The table lists standard entropy, SΘ, values.
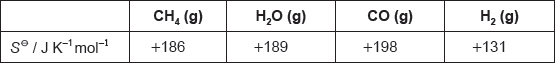
Calculate the standard entropy change for the reaction, ΔSΘ, in J K−1.
CH4(g) + H2O(g) → 3H2(g) + CO(g)
Calculate the standard free energy change, ΔGΘ, in kJ, for the reaction at 298 K using your answer to (b)(ii).
Determine the temperature, in K, above which the reaction becomes spontaneous.
Dinitrogen monoxide, N2O, causes depletion of ozone in the stratosphere.
Different sources of N2O have different ratios of 14N : 15N.
The Lewis (electron dot) structure of the dinitrogen monoxide molecule can be represented as:
Outline why ozone in the stratosphere is important.
Dinitrogen monoxide in the stratosphere is converted to nitrogen monoxide, NO (g).
Write two equations to show how NO (g) catalyses the decomposition of ozone.
State one analytical technique that could be used to determine the ratio of 14N : 15N.
A sample of gas was enriched to contain 2 % by mass of 15N with the remainder being 14N.
Calculate the relative molecular mass of the resulting N2O.
Predict, giving two reasons, how the first ionization energy of 15N compares with that of 14N.
Explain why the first ionization energy of nitrogen is greater than both carbon and oxygen.
Nitrogen and carbon:
Nitrogen and oxygen:
State what the presence of alternative Lewis structures shows about the nature of the bonding in the molecule.
State, giving a reason, the shape of the dinitrogen monoxide molecule.
Deduce the hybridization of the central nitrogen atom in the molecule.
This question is about sodium and its compounds.
The Born-Haber cycle for sodium oxide is shown (not to scale).
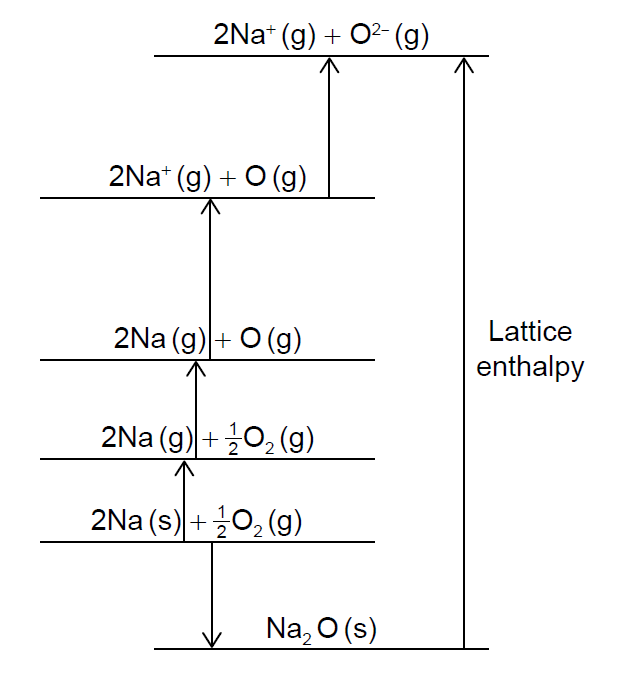
Sodium peroxide is used in diving apparatus to produce oxygen from carbon dioxide.
2Na2O2 (s) + 2CO2 (g) → 2Na2CO3 (s) + O2 (g)
Plot the relative values of the first four ionization energies of sodium.
Outline why the alkali metals (group 1) have similar chemical properties.
Describe the structure and bonding in solid sodium oxide.
Calculate values for the following changes using section 8 of the data booklet.
ΔHatomisation (Na) = 107 kJ mol−1
ΔHatomisation (O) = 249 kJ mol−1
O2(g) → O2- (g):
Na (s) → Na+ (g):
The standard enthalpy of formation of sodium oxide is −414 kJ mol−1. Determine the lattice enthalpy of sodium oxide, in kJ mol−1, using section 8 of the data booklet and your answers to (d)(i).
(If you did not get answers to (d)(i), use +850 kJ mol−1 and +600 kJ mol−1 respectively, but these are not the correct answers.)
Justify why K2O has a lower lattice enthalpy (absolute value) than Na2O.
Write equations for the separate reactions of solid sodium oxide and solid phosphorus(V) oxide with excess water and differentiate between the solutions formed.
Sodium oxide, Na2O:
Phosphorus(V) oxide, P4O10:
Differentiation:
Sodium peroxide, Na2O2, is formed by the reaction of sodium oxide with oxygen.
2Na2O (s) + O2 (g) → 2Na2O2 (s)
Calculate the percentage yield of sodium peroxide if 5.00g of sodium oxide produces 5.50g of sodium peroxide.
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
Outline why bond enthalpy values are not valid in calculations such as that in (g)(i).
An allotrope of molecular oxygen is ozone. Compare, giving a reason, the bond enthalpies of the O to O bonds in O2 and O3.
Outline why a real gas differs from ideal behaviour at low temperature and high pressure.
The reaction of sodium peroxide with excess water produces hydrogen peroxide and one other sodium compound. Suggest the formula of this compound.
State the oxidation number of carbon in sodium carbonate, Na2CO3.
Ethane-1,2-diol, HOCH2CH2OH, reacts with thionyl chloride, SOCl2, according to the reaction below.
HOCH2CH2OH (l) + 2SOCl2 (l) → ClCH2CH2Cl (l) + 2SO2 (g) + 2HCl (g)
Calculate the standard enthalpy change for this reaction using the following data.
Calculate the standard entropy change for this reaction using the following data.
The standard free energy change, ΔGθ, for the above reaction is –103 kJ mol–1 at 298 K.
Suggest why ΔGθ has a large negative value considering the sign of ΔHθ in part (a).
White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.
Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
Calculate the entropy change, ΔS, in J K−1 mol−1, for this reaction.
Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for Selected Substances https://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances# page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
Calculate the Gibbs free energy change (ΔG), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
State the equilibrium constant expression, Kc, for this reaction.
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.
Organic chemistry can be used to synthesize a variety of products.
Combustion analysis of an unknown organic compound indicated that it contained only carbon, hydrogen and oxygen.
Several compounds can be synthesized from but-2-ene. Draw the structure of the final product for each of the following chemical reactions.
Determine the change in enthalpy, ΔH, for the combustion of but-2-ene, using section 11 of the data booklet.
CH3CH=CHCH3 (g) + 6O2 (g) → 4CO2 (g) + 4H2O (g)
State the hybridization of the carbon I and II atoms in but-2-ene.
Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
Sketch the mechanism for the reaction of 2-methylbut-2-ene with hydrogen bromide using curly arrows.
Explain why the major organic product is 2-bromo-2-methylbutane and not 2-bromo-3-methylbutane.
Deduce two features of this molecule that can be obtained from the mass spectrum. Use section 28 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
Identify the bond responsible for the absorption at A in the infrared spectrum. Use section 26 of the data booklet.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce
on behalf of the United States of America. All rights reserved.
Deduce the identity of the unknown compound using the previous information, the 1H NMR spectrum and section 27 of the data booklet.
SDBS, National Institute of Advanced Industrial Science and Technology (AIST).
Draw the stereoisomers of butan-2-ol using wedge-dash type representations.
Outline how two enantiomers can be distinguished using a polarimeter.
Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
Two electrolysis cells were assembled using graphite electrodes and connected in series as shown.
Copper(I) chloride undergoes a disproportionation reaction, producing copper(II) chloride and copper.
2Cu+ (aq) → Cu (s) + Cu2+ (aq)
Dilute copper(II) chloride solution is light blue, while copper(I) chloride solution is colourless.
State the electron configuration of the Cu+ ion.
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label Ea (cat).
Explain how the catalyst increases the rate of the reaction.
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•H2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of . The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of .
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
Write the half-equation for the formation of gas bubbles at electrode 1.
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
Deduce the half-equation for the formation of the gas identified in (c)(iii).
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24 of the data booklet.
Comment on the spontaneity of the disproportionation reaction at 298 K.
Calculate the standard Gibbs free energy change, ΔGθ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
Deduce, giving a reason, the sign of the standard enthalpy change, ΔHθ, for the disproportionation reaction at 298 K.
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
Deduce why the Cu(I) solution is colourless.
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
3.26 g of iron powder are added to 80.0 cm3 of 0.200 mol dm−3 copper(II) sulfate solution. The following reaction occurs:
Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
Determine the limiting reactant showing your working.
The mass of copper obtained experimentally was 0.872 g. Calculate the percentage yield of copper.
The reaction was carried out in a calorimeter. The maximum temperature rise of the solution was 7.5 °C.
Calculate the enthalpy change, ΔH, of the reaction, in kJ, assuming that all the heat released was absorbed by the solution. Use sections 1 and 2 of the data booklet.
State another assumption you made in (b)(i).
The only significant uncertainty is in the temperature measurement.
Determine the absolute uncertainty in the calculated value of ΔH if the uncertainty in the temperature rise was ±0.2 °C.
Sketch a graph of the concentration of iron(II) sulfate, FeSO4, against time as the reaction proceeds.
Outline how the initial rate of reaction can be determined from the graph in part (c)(i).
Explain, using the collision theory, why replacing the iron powder with a piece of iron of the same mass slows down the rate of the reaction.
A student electrolyzed aqueous iron(II) sulfate, FeSO4 (aq), using platinum electrodes. State half-equations for the reactions at the electrodes, using section 24 of the data booklet.
Propene is an important starting material for many products. The following shows some compounds which can be made from propene, C3H6.
Propene (C3H6) → C3H7Cl → C3H8O → C3H6O
Consider the conversion of propene to C3H7Cl.
An experiment was carried out to determine the order of reaction between one of the isomers of C3H7Cl and aqueous sodium hydroxide. The following results were obtained.
State the type of reaction.
State the IUPAC name of the major product.
Outline why it is the major product.
Write an equation for the reaction of the major product with aqueous sodium hydroxide to produce a C3H8O compound, showing structural formulas.
Determine the rate expression from the results, explaining your method.
Deduce the type of mechanism for the reaction of this isomer of C3H7Cl with aqueous sodium hydroxide.
Sketch the mechanism using curly arrows to represent the movement of electrons.
Write an equation for the complete combustion of the compound C3H8O formed in (a)(iv).
Determine the enthalpy of combustion of this compound, in kJ mol−1, using data from section 11 of the data booklet.
State the reagents for the conversion of the compound C3H8O formed in (a)(iv) into C3H6O.
Explain why the compound C3H8O, produced in (a)(iv), has a higher boiling point than compound C3H6O, produced in d(i).
Explain why the 1H NMR spectrum of C3H6O, produced in (d)(i), shows only one signal.
Propene is often polymerized. Draw a section of the resulting polymer, showing two repeating units.
Magnetite, Fe3O4, is another ore of iron that contains both Fe2+ and Fe3+.
Iron exists as several isotopes.
Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
State the type of spectroscopy that could be used to determine their relative abundances.
State the number of protons, neutrons and electrons in each species.
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
A voltaic cell is set up between the Fe2+ (aq) | Fe (s) and Fe3+ (aq) | Fe2+ (aq) half-cells.
Deduce the equation and the cell potential of the spontaneous reaction. Use section 24 of the data booklet.
The figure shows an apparatus that could be used to electroplate iron with zinc. Label the figure with the required substances.
Outline why, unlike typical transition metals, zinc compounds are not coloured.
Transition metals like iron can form complex ions. Discuss the bonding between transition metals and their ligands in terms of acid-base theory.
Ethane-1,2-diol, HOCH2CH2OH, has a wide variety of uses including the removal of ice from aircraft and heat transfer in a solar cell.
(i) Calculate ΔHθ, in kJ, for this similar reaction below using data from section 12 of the data booklet. of HOCH2CH2OH(l) is –454.8kJmol-1.
2CO (g) + 3H2 (g) HOCH2CH2OH (l)
(ii) Deduce why the answers to (a)(iii) and (b)(i) differ.
(iii) ΔSθ for the reaction in (b)(i) is –620.1JK-1. Comment on the decrease in entropy.
(iv) Calculate the value of ΔGθ, in kJ, for this reaction at 298 K using your answer to (b)(i). (If you did not obtain an answer to (b)(i), use –244.0 kJ, but this is not the correct value.)
(v) Comment on the statement that the reaction becomes less spontaneous as temperature is increased.
Predict the 1HNMR data for ethanedioic acid and ethane-1,2-diol by completing the table.
A student titrated two acids, hydrochloric acid, HCl (aq) and ethanoic acid, CH3COOH (aq), against 50.0 cm3 of 0.995 mol dm–3 sodium hydroxide, NaOH (aq), to determine their concentration. The temperature of the reaction mixture was measured after each acid addition and plotted against the volume of each acid.
Using the graph, estimate the initial temperature of the solutions.
Determine the maximum temperature reached in each experiment by analysing the graph.
Suggest why the enthalpy change of neutralization of CH3COOH is less negative than that of HCl.