
HL Paper 2
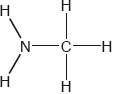
Two hydrides of nitrogen are ammonia and hydrazine, . One derivative of ammonia is methanamine whose molecular structure is shown below.
Hydrazine is used to remove oxygen from water used to generate steam or hot water.
The concentration of dissolved oxygen in a sample of water is .
Estimate the H−N−H bond angle in methanamine using VSEPR theory.
State the electron domain geometry around the nitrogen atom and its hybridization in methanamine.
Ammonia reacts reversibly with water.
Explain the effect of adding ions on the position of the equilibrium.
Hydrazine reacts with water in a similar way to ammonia. (The association of a molecule of hydrazine with a second H+ is so small it can be neglected.)
Calculate the pH of a solution of hydrazine.
Suggest a suitable indicator for the titration of hydrazine solution with dilute sulfuric acid using section 22 of the data booklet.
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
Determine the enthalpy change of reaction, , in kJ, when 1.00 mol of gaseous hydrazine decomposes to its elements. Use bond enthalpy values in section 11 of the data booklet.
The standard enthalpy of formation of is . Calculate the enthalpy of vaporization, , of hydrazine in . (If you did not get an answer to (f), use but this is not the correct answer.)
Calculate, showing your working, the mass of hydrazine needed to remove all the dissolved oxygen from of the sample.
Calculate the volume, in , of nitrogen formed under SATP conditions. (The volume of 1 mol of gas = at SATP.)
Iron(II) disulfide, FeS2, has been mistaken for gold.
State the full electronic configuration of Fe2+.
Explain why there is a large increase from the 8th to the 9th ionization energy of iron.
Calculate the oxidation state of sulfur in iron(II) disulfide, FeS2.
Describe the bonding in iron, Fe (s).
Nickel catalyses the conversion of propanone to propan-2-ol.
Outline how a catalyst increases the rate of reaction.
Explain why an increase in temperature increases the rate of reaction.
Discuss, referring to intermolecular forces present, the relative volatility of propanone and propan-2-ol.
The diagram shows an unlabelled voltaic cell for the reaction
Label the diagram with the species in the equation.
Calculate the standard cell potential, in , for the cell at . Use section 24 of the data booklet
Calculate the standard free energy change, , in , for the cell using sections 1 and 2 of the data booklet.
Suggest a metal that could replace nickel in a new half-cell and reverse the electron flow. Use section 25 of the data booklet.
Describe the bonding in metals.
Nickel alloys are used in aircraft gas turbines. Suggest a physical property altered by the addition of another metal to nickel.
The equations show steps in the formation and decomposition of ozone in the stratosphere, some of which absorb ultraviolet light.
Step 1 O2 → 2O•
Step 2 O• + O2 → O3
Step 3 O3 → O• + O2
Step 4 O• + O3 → 2O2
Draw the Lewis structures of oxygen, O2, and ozone, O3.
Outline why both bonds in the ozone molecule are the same length and predict the bond length in the ozone molecule. Refer to section 10 of the data booklet.
Reason:
Length:
Predict the bond angle in the ozone molecule.
Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone layer affect radiation reaching the Earth’s surface.
Identify the steps which absorb ultraviolet light.
Determine, showing your working, the wavelength, in m, of ultraviolet light absorbed by a single molecule in one of these steps. Use sections 1, 2 and 11 of the data booklet.
Ozone depletion is catalysed by nitrogen monoxide, NO, which is produced in aircraft and motor vehicle engines, and has the following Lewis structure.
Show how nitrogen monoxide catalyses the decomposition of ozone, including equations in your answer.
Titanium and vanadium are consecutive elements in the first transition metal series.
reacts with water and the resulting titanium(IV) oxide can be used as a smoke screen.
Describe the bonding in metals.
Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the following data:
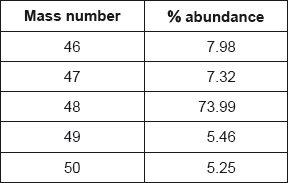
Calculate the relative atomic mass of titanium to two decimal places.
State the number of protons, neutrons and electrons in the atom.
State the full electron configuration of the ion.
Suggest why the melting point of vanadium is higher than that of titanium.
Sketch a graph of the first six successive ionization energies of vanadium on the axes provided.
Explain why an aluminium-titanium alloy is harder than pure aluminium.
Describe, in terms of the electrons involved, how the bond between a ligand and a central metal ion is formed.
Outline why transition metals form coloured compounds.
State the type of bonding in potassium chloride which melts at 1043 K.
A chloride of titanium, , melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
Formulate an equation for this reaction.
Suggest one disadvantage of using this smoke in an enclosed space.
Iron may be extracted from iron (II) sulfide, FeS.
Iron (II) sulfide, FeS, is ionically bonded.
The first step in the extraction of iron from iron (II) sulfide is to roast it in air to form iron (III) oxide and sulfur dioxide.
Outline why metals, like iron, can conduct electricity.
Justify why sulfur is classified as a non-metal by giving two of its chemical properties.
Sketch the first eight successive ionisation energies of sulfur.
Describe the bonding in this type of solid.
State a technique that could be used to determine the crystal structure of the solid compound.
State the full electron configuration of the sulfide ion.
Outline, in terms of their electronic structures, why the ionic radius of the sulfide ion is greater than that of the oxide ion.
Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
Write the equation for this reaction.
Deduce the change in the oxidation state of sulfur.
Suggest why this process might raise environmental concerns.
Explain why the addition of small amounts of carbon to iron makes the metal harder.
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) (H2N)2CO(g) + H2O(g) ΔH < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
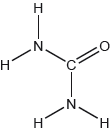
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, Kc.
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.
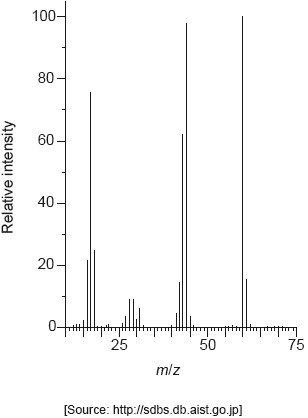
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.
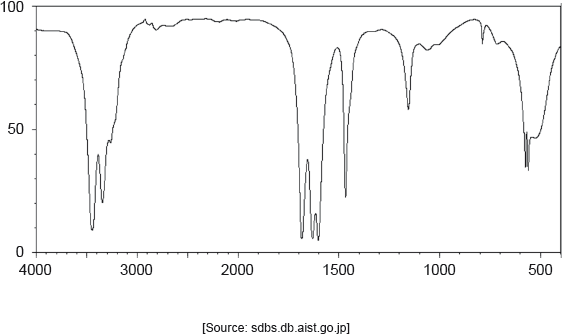
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
Some physical properties of molecular substances result from the different types of forces between their molecules.
Resonance structures exist when a molecule can be represented by more than one Lewis structure.
Carbon dioxide can be represented by at least two resonance structures, I and II.

Calculate the formal charge on each oxygen atom in the two structures.
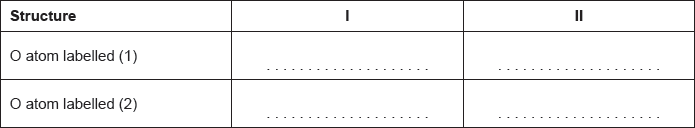
Deduce, giving a reason, the more likely structure.
Absorption of UV light in the ozone layer causes the dissociation of oxygen and ozone.
Identify, in terms of bonding, the molecule that requires a longer wavelength to dissociate.
Carbon and silicon are elements in group 14.
Explain why CO2 is a gas but SiO2 is a solid at room temperature.
Bromine can form the bromate(V) ion, BrO3−.
State the electron configuration of a bromine atom.
Sketch the orbital diagram of the valence shell of a bromine atom (ground state) on the energy axis provided. Use boxes to represent orbitals and arrows to represent electrons.
Draw two Lewis (electron dot) structures for BrO3−.
Determine the preferred Lewis structure based on the formal charge on the bromine atom, giving your reasons.
Predict, using the VSEPR theory, the geometry of the BrO3− ion and the O−Br−O bond angles.
Bromate(V) ions act as oxidizing agents in acidic conditions to form bromide ions.
Deduce the half-equation for this reduction reaction.
Bromate(V) ions oxidize iron(II) ions, Fe2+, to iron(III) ions, Fe3+.
Deduce the equation for this redox reaction.
Calculate the standard Gibbs free energy change, ΔGΘ, in J, of the redox reaction in (ii), using sections 1 and 24 of the data booklet.
EΘ (BrO3− / Br−) = +1.44 V
State and explain the magnetic property of iron(II) and iron(III) ions.
Calcium carbide, CaC2, is an ionic solid.
Describe the nature of ionic bonding.
Describe how the relative atomic mass of a sample of calcium could be determined from its mass spectrum.
When calcium compounds are introduced into a gas flame a red colour is seen; sodium compounds give a yellow flame. Outline the source of the colours and why they are different.
Suggest two reasons why solid calcium has a greater density than solid potassium.
Outline why solid calcium is a good conductor of electricity.
Sketch a graph of the first six ionization energies of calcium.
Calcium carbide reacts with water to form ethyne and calcium hydroxide.
CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(aq)
Estimate the pH of the resultant solution.
Describe how sigma (σ) and pi () bonds are formed.
Deduce the number of σ and bonds in a molecule of ethyne.
Organomagnesium compounds can react with carbonyl compounds. One overall equation is:
Compound B can also be prepared by reacting an alkene with water.
Iodomethane is used to prepare CH3Mg. It can also be converted into methanol:
CH3 + HO– → CH3OH + –
State the name of Compound B, applying International Union of Pure and Applied Chemistry (IUPAC) rules.
Compound A and Compound B are both liquids at room temperature and pressure. Identify the strongest intermolecular force between molecules of Compound A.
State the number of (sigma) and (pi) bonds in Compound A.
Deduce the hybridization of the central carbon atom in Compound A.
Identify the isomer of Compound B that exists as optical isomers (enantiomers).
Draw the structural formula of the alkene required.
Explain why the reaction produces more (CH3)3COH than (CH3)2CHCH2OH.
Deduce the structural formula of the repeating unit of the polymer formed from this alkene.
Deduce what would be observed when Compound B is warmed with acidified aqueous potassium dichromate (VI).
Identify the type of reaction.
Outline the requirements for a collision between reactants to yield products.
Explain the mechanism of the reaction using curly arrows to represent the movement of electron pairs.
The polarity of the carbon–halogen bond, C–X, facilitates attack by HO–.
Outline, giving a reason, how the bond polarity changes going down group 17.
Both vinegar (a dilute aqueous solution of ethanoic acid) and bleach are used as cleaning agents.
Bleach reacts with ammonia, also used as a cleaning agent, to produce the poisonous compound chloramine, NH2Cl.
Outline why ethanoic acid is classified as a weak acid.
A solution of bleach can be made by reacting chlorine gas with a sodium hydroxide solution.
Cl2 (g) + 2NaOH (aq) ⇌ NaOCl (aq) + NaCl (aq) + H2O (l)
Suggest, with reference to Le Châtelier’s principle, why it is dangerous to mix vinegar and bleach together as cleaners.
Draw a Lewis (electron dot) structure of chloramine.
State the hybridization of the nitrogen atom in chloramine.
Deduce the molecular geometry of chloramine and estimate its H–N–H bond angle.
Molecular geometry:
H–N–H bond angle:
State the type of bond formed when chloramine is protonated.
Sketch a graph of pH against volume of hydrochloric acid added to ammonia solution, showing how you would determine the pKa of the ammonium ion.
Suggest a suitable indicator for the titration, using section 22 of the data booklet.
Explain, using two equations, how an equimolar solution of ammonia and ammonium ions acts as a buffer solution when small amounts of acid or base are added.
Bonds can be formed in many ways.
The equilibrium for a mixture of NO2 and N2O4 gases is represented as:
2NO2(g) N2O4(g)
At 100°C, the equilibrium constant, Kc, is 0.21.
Bonds can be formed in many ways.
Discuss the bonding in the resonance structures of ozone.
Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen atom.
The first six ionization energies, in kJ mol–1, of an element are given below.

Explain the large increase in ionization energy from IE3 to IE4.
At a given time, the concentration of NO2(g) and N2O4(g) were 0.52 and respectively.
Deduce, showing your reasoning, if the forward or the reverse reaction is favoured at this time.
Comment on the value of ΔG when the reaction quotient equals the equilibrium constant, Q = K.
Cobalt forms the transition metal complex [Co(NH3)4 (H2O)Cl]Br.
Trends in physical and chemical properties are useful to chemists.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group whereas the melting points of the group 17 elements (F → I) increase down the group.
State the shape of the complex ion.
Deduce the charge on the complex ion and the oxidation state of cobalt.
Describe, in terms of acid-base theories, the type of reaction that takes place between the cobalt ion and water to form the complex ion.
Ethyne, C2H2, reacts with oxygen in welding torches.
Ethyne reacts with steam.
C2H2 (g) + H2O (g) → C2H4O (g)
Two possible products are:
Product B, CH3CHO, can also be synthesized from ethanol.
Write an equation for the complete combustion of ethyne.
Deduce the Lewis (electron dot) structure of ethyne.
Compare, giving a reason, the length of the bond between the carbon atoms in ethyne with that in ethane, C2H6.
Identify the type of interaction that must be overcome when liquid ethyne vaporizes.
State the name of product B, applying IUPAC rules.
Determine the enthalpy change for the reaction, in kJ, to produce A using section 11 of the data booklet.
The enthalpy change for the reaction to produce B is −213 kJ.
Predict, giving a reason, which product is the most stable.
The IR spectrum and low resolution 1H NMR spectrum of the actual product formed are shown.
Deduce whether the product is A or B, using evidence from these spectra together with sections 26 and 27 of the data booklet.
Identity of product:
One piece of evidence from IR:
One piece of evidence from 1H NMR:
Deduce the splitting pattern you would expect for the signals in a high resolution 1H NMR spectrum.
2.3 ppm:
9.8 ppm:
Suggest the reagents and conditions required to ensure a good yield of product B.
Reagents:
Conditions:
Deduce the average oxidation state of carbon in product B.
Explain why product B is water soluble.
Sulfur trioxide is produced from sulfur dioxide.
2SO2 (g) + O2 (g) 2SO3 (g) ΔH = −196 kJ mol−1
The reaction between sulfur dioxide and oxygen can be carried out at different temperatures.
Outline, giving a reason, the effect of a catalyst on a reaction.
On the axes, sketch Maxwell–Boltzmann energy distribution curves for the reacting species at two temperatures T1 and T2, where T2 > T1.
Explain the effect of increasing temperature on the yield of SO3.
Draw the Lewis structure of SO3.
Explain the electron domain geometry of SO3.
State the product formed from the reaction of SO3 with water.
State the meaning of a strong Brønsted–Lowry acid.
The properties of elements can be predicted from their position in the periodic table.
Explain why Si has a smaller atomic radius than Al.
Explain why the first ionization energy of sulfur is lower than that of phosphorus.
State the condensed electron configurations for Cr and Cr3+.
Describe metallic bonding and how it contributes to electrical conductivity.
Deduce, giving a reason, which complex ion [Cr(CN)6]3− or [Cr(OH)6]3− absorbs higher energy light. Use section 15 of the data booklet.
[Cr(OH)6]3− forms a green solution. Estimate a wavelength of light absorbed by this complex, using section 17 of the data booklet.
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur tetrafluoride, SF4, and sulfur dichloride, SCl2.
Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.
Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
Calculate the entropy change, ΔS, in J K−1 mol−1, for this reaction.
Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for Selected Substances https://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances# page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
Calculate the Gibbs free energy change (ΔG), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
State the equilibrium constant expression, Kc, for this reaction.
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.
Magnetite, Fe3O4, is another ore of iron that contains both Fe2+ and Fe3+.
Iron exists as several isotopes.
Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
State the type of spectroscopy that could be used to determine their relative abundances.
State the number of protons, neutrons and electrons in each species.
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
A voltaic cell is set up between the Fe2+ (aq) | Fe (s) and Fe3+ (aq) | Fe2+ (aq) half-cells.
Deduce the equation and the cell potential of the spontaneous reaction. Use section 24 of the data booklet.
The figure shows an apparatus that could be used to electroplate iron with zinc. Label the figure with the required substances.
Outline why, unlike typical transition metals, zinc compounds are not coloured.
Transition metals like iron can form complex ions. Discuss the bonding between transition metals and their ligands in terms of acid-base theory.
The concentration of a solution of a weak acid, such as ethanedioic acid, can be determined
by titration with a standard solution of sodium hydroxide, NaOH (aq).
5.00 g of an impure sample of hydrated ethanedioic acid, (COOH)2•2H2O, was dissolved in water to make 1.00 dm3 of solution. 25.0 cm3 samples of this solution were titrated against a 0.100 mol dm-3 solution of sodium hydroxide using a suitable indicator.
(COOH)2 (aq) + 2NaOH (aq) → (COONa)2 (aq) + 2H2O (l)
The mean value of the titre was 14.0 cm3.
(i) Suggest a suitable indicator for this titration. Use section 22 of the data booklet.
(ii) Calculate the amount, in mol, of NaOH in 14.0 cm3 of 0.100 mol dm-3 solution.
(iii) Calculate the amount, in mol, of ethanedioic acid in each 25.0 cm3 sample.
(iv) Determine the percentage purity of the hydrated ethanedioic acid sample.
Draw the Lewis (electron dot) structure of the ethanedioate ion, –OOCCOO–.
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
Explain how ethanedioate ions act as ligands.
Biochemical oxygen demand (BOD) can be determined by the Winkler Method.
A 25.00 cm3 sample of water was treated according to the Winkler Method.
Step I: 2Mn2+ (aq) + O2 (g) + 4OH− (aq) → 2MnO2 (s) + 2H2O (l)
Step II: MnO2 (s) + 2I− (aq) + 4H+ (aq) → Mn2+ (aq) + I2 (aq) + 2H2O (l)
Step III: 2S2O32− (aq) + I2 (aq) → 2I− (aq) + S4O62− (aq)
The iodine produced was titrated with 37.50 cm3 of 5.000 × 10−4 mol dm−3 Na2S2O3.
Outline what is measured by BOD.
A student dissolved 0.1240 ± 0.0001 g of Na2S2O3 to make 1000.0 ± 0.4 cm3 of solution to use in the Winkler Method.
Determine the percentage uncertainty in the molar concentration.
Calculate the amount, in moles of Na2S2O3 used in the titration.
Deduce the mole ratio of O2 consumed in step I to S2O32− used in step III.
Calculate the concentration of dissolved oxygen, in mol dm−3, in the sample.
The three steps of the Winkler Method are redox reactions.
Deduce the reduction half-equation for step II.
Suggest a reason that the Winkler Method used to measure biochemical oxygen demand (BOD) must be done at constant temperature.
Lewis (electron dot) structures are useful models.
Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce the molecular geometry of each species including bond angles.
Predict whether the molecules PF3 and PF5 are polar or non-polar.
State the type of hybridization shown by the phosphorus atom in PF3.
Benzoic acid, C6H5COOH, is another derivative of benzene.
Identify the wavenumber of one peak in the IR spectrum of benzoic acid, using section 26 of the data booklet.
Identify the spectroscopic technique that is used to measure the bond lengths in solid benzoic acid.
Outline one piece of physical evidence for the structure of the benzene ring.
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
Outline why both C to O bonds in the conjugate base are the same length and suggest a value for them. Use section 10 of the data booklet.
The pH of an aqueous solution of benzoic acid at 298 K is 2.95. Determine the concentration of hydroxide ions in the solution, using section 2 of the data booklet.
Formulate the equation for the complete combustion of benzoic acid in oxygen using only integer coefficients.
The combustion reaction in (f)(ii) can also be classed as redox. Identify the atom that is oxidized and the atom that is reduced.
Suggest how benzoic acid, Mr = 122.13, forms an apparent dimer, Mr = 244.26, when dissolved in a non-polar solvent such as hexane.
State the reagent used to convert benzoic acid to phenylmethanol (benzyl alcohol), C6H5CH2OH.
Carbon forms many compounds.
C60 and diamond are allotropes of carbon.
Chlorine reacts with methane.
CH4 (g) + Cl2 (g) → CH3Cl (g) + HCl (g)
Outline two differences between the bonding of carbon atoms in C60 and diamond.
Explain why C60 and diamond sublime at different temperatures and pressures.
State two features showing that propane and butane are members of the same homologous series.
Describe a test and the expected result to indicate the presence of carbon–carbon double bonds.
Draw the full structural formula of (Z)-but-2-ene.
Write the equation for the reaction between but-2-ene and hydrogen bromide.
State the type of reaction.
Suggest two differences in the 1H NMR of but-2-ene and the organic product from (d)(ii).
Predict, giving a reason, the major product of reaction between but-1-ene and steam.
Explain the mechanism of the reaction between 1-bromopropane, CH3CH2CH2Br, and aqueous sodium hydroxide, NaOH (aq), using curly arrows to represent the movement of electron pairs.
Deduce the splitting pattern in the 1H NMR spectrum for 1-bromopropane.
Calculate the enthalpy change of the reaction, ΔH, using section 11 of the data booklet.
Draw and label an enthalpy level diagram for this reaction.
Oxygen exists as two allotropes, diatomic oxygen, O2, and ozone, O3.
Draw a Lewis (electron dot) structure for ozone.
Discuss the relative length of the two O−O bonds in ozone.
Explain why there are frequencies of UV light that will dissociate O3 but not O2.
Explain, using equations, how the presence of results in a chain reaction that decreases the concentration of ozone in the stratosphere.
Compound A is in equilibrium with compound B.
Predict the electron domain and molecular geometries around the oxygen atom of molecule A using VSEPR
State the type of hybridization shown by the central carbon atom in molecule B.
State the number of sigma () and pi () bonds around the central carbon atom in molecule B.
The IR spectrum of one of the compounds is shown:
COBLENTZ SOCIETY. Collection © 2018 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Deduce, giving a reason, the compound producing this spectrum.
Compound A and B are isomers. Draw two other structural isomers with the formula .
The equilibrium constant, , for the conversion of A to B is in water at .
Deduce, giving a reason, which compound, A or B, is present in greater concentration when equilibrium is reached.
Calculate the standard Gibbs free energy change, , in , for the reaction (A to B) at . Use sections 1 and 2 of the data booklet.
Propanone can be synthesized in two steps from propene. Suggest the synthetic route including all the necessary reactants and steps.
Propanone can be synthesized in two steps from propene.
Suggest why propanal is a minor product obtained from the synthetic route in (g)(i).
The overall equation for the production of hydrogen cyanide, HCN, is shown below.
CH4 (g) + NH3 (g) +O2 (g) → HCN (g) + 3H2O (g)
State why NH3 is a Lewis base.
Calculate the pH of a 1.00 × 10−2 mol dm−3 aqueous solution of ammonia.
pKb = 4.75 at 298 K.
Justify whether a 1.0 dm3 solution made from 0.10 mol NH3 and 0.20 mol HCl will form a buffer solution.
Sketch the shape of one sigma () and one pi () bond.
Identify the number of sigma and pi bonds in HCN.
State the hybridization of the carbon atom in HCN.
Suggest why hydrogen chloride, HCl, has a lower boiling point than hydrogen cyanide, HCN.
Explain why transition metal cyanide complexes are coloured.
Carbonated water is produced when carbon dioxide is dissolved in water under pressure. The following equilibria are established.
Equilibrium (1) CO2 (g) CO2 (aq)
Equilibrium (2) CO2 (aq) + H2O (l) H+ (aq) + HCO3− (aq)
Carbon dioxide acts as a weak acid.
Soda water has sodium hydrogencarbonate, NaHCO3, dissolved in the carbonated water.
Distinguish between a weak and strong acid.
Weak acid:
Strong acid:
The hydrogencarbonate ion, produced in Equilibrium (2), can also act as an acid.
State the formula of its conjugate base.
When a bottle of carbonated water is opened, these equilibria are disturbed.
State, giving a reason, how a decrease in pressure affects the position of Equilibrium (1).
At 298 K the concentration of aqueous carbon dioxide in carbonated water is 0.200 mol dm−3 and the pKa for Equilibrium (2) is 6.36.
Calculate the pH of carbonated water.
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
Predict, referring to Equilibrium (2), how the added sodium hydrogencarbonate affects the pH.(Assume pressure and temperature remain constant.)
100.0cm3 of soda water contains 3.0 × 10−2g NaHCO3.
Calculate the concentration of NaHCO3 in mol dm−3.
The uncertainty of the 100.0cm3 volumetric flask used to make the solution was ±0.6cm3.
Calculate the maximum percentage uncertainty in the mass of NaHCO3 so that the concentration of the solution is correct to ±1.0 %.
The reaction of the hydroxide ion with carbon dioxide and with the hydrogencarbonate ion can be represented by Equations 3 and 4.
Equation (3) OH− (aq) + CO2 (g) → HCO3− (aq)
Equation (4) OH− (aq) + HCO3− (aq) → H2O (l) + CO32− (aq)
Discuss how these equations show the difference between a Lewis base and a Brønsted–Lowry base.
Equation (3):
Equation (4):
Aqueous sodium hydrogencarbonate has a pH of approximately 7 at 298 K.
Sketch a graph of pH against volume when 25.0cm3 of 0.100 mol dm−3 NaOH (aq) is gradually added to 10.0cm3 of 0.0500 mol dm−3 NaHCO3 (aq).
This question is about sodium and its compounds.
The Born-Haber cycle for sodium oxide is shown (not to scale).
Sodium peroxide is used in diving apparatus to produce oxygen from carbon dioxide.
2Na2O2 (s) + 2CO2 (g) → 2Na2CO3 (s) + O2 (g)
Plot the relative values of the first four ionization energies of sodium.
Outline why the alkali metals (group 1) have similar chemical properties.
Describe the structure and bonding in solid sodium oxide.
Calculate values for the following changes using section 8 of the data booklet.
ΔHatomisation (Na) = 107 kJ mol−1
ΔHatomisation (O) = 249 kJ mol−1
O2(g) → O2- (g):
Na (s) → Na+ (g):
The standard enthalpy of formation of sodium oxide is −414 kJ mol−1. Determine the lattice enthalpy of sodium oxide, in kJ mol−1, using section 8 of the data booklet and your answers to (d)(i).
(If you did not get answers to (d)(i), use +850 kJ mol−1 and +600 kJ mol−1 respectively, but these are not the correct answers.)
Justify why K2O has a lower lattice enthalpy (absolute value) than Na2O.
Write equations for the separate reactions of solid sodium oxide and solid phosphorus(V) oxide with excess water and differentiate between the solutions formed.
Sodium oxide, Na2O:
Phosphorus(V) oxide, P4O10:
Differentiation:
Sodium peroxide, Na2O2, is formed by the reaction of sodium oxide with oxygen.
2Na2O (s) + O2 (g) → 2Na2O2 (s)
Calculate the percentage yield of sodium peroxide if 5.00g of sodium oxide produces 5.50g of sodium peroxide.
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
Outline why bond enthalpy values are not valid in calculations such as that in (g)(i).
An allotrope of molecular oxygen is ozone. Compare, giving a reason, the bond enthalpies of the O to O bonds in O2 and O3.
Outline why a real gas differs from ideal behaviour at low temperature and high pressure.
The reaction of sodium peroxide with excess water produces hydrogen peroxide and one other sodium compound. Suggest the formula of this compound.
State the oxidation number of carbon in sodium carbonate, Na2CO3.
Nitric acid is usually produced by the oxidation of ammonia.
A mixture of nitric acid and sulfuric acid can be used to convert benzene to nitrobenzene, C6H5NO2.
Draw arrows in the boxes to represent the electron configuration of a nitrogen atom.
Deduce a Lewis (electron dot) structure of the nitric acid molecule, HNO3, that obeys the octet rule, showing any non-zero formal charges on the atoms.
Explain the relative lengths of the three bonds between N and O in nitric acid.
State a technique used to determine the length of the bonds between N and O in solid HNO3.
Write an equation for the reaction between the acids to produce the electrophile, NO2+.
Draw the structural formula of the carbocation intermediate produced when this electrophile attacks benzene.
Deduce the number of signals that you would expect in the 1H NMR spectrum of nitrobenzene and the relative areas of these.
Butanoic acid, CH3CH2CH2COOH, is a weak acid and ethylamine, CH3CH2NH2, is a weak base.
State the equation for the reaction of each substance with water.
Draw a diagram showing the delocalization of electrons in the conjugate base of butanoic acid.
Deduce the average oxidation state of carbon in butanoic acid.
A 0.250 mol dm−3 aqueous solution of butanoic acid has a concentration of hydrogen ions, [H+], of 0.00192 mol dm−3. Calculate the concentration of hydroxide ions, [OH−], in the solution at 298 K.
Determine the pH of a 0.250 mol dm−3 aqueous solution of ethylamine at 298 K, using section 21 of the data booklet.
Sketch the pH curve for the titration of 25.0 cm3 of ethylamine aqueous solution with 50.0 cm3 of butanoic acid aqueous solution of equal concentration. No calculations are required.
Explain why butanoic acid is a liquid at room temperature while ethylamine is a gas at room temperature.
State a suitable reagent for the reduction of butanoic acid.
Deduce the product of the complete reduction reaction in (e)(i).
Dinitrogen monoxide, N2O, causes depletion of ozone in the stratosphere.
Different sources of N2O have different ratios of 14N : 15N.
The Lewis (electron dot) structure of the dinitrogen monoxide molecule can be represented as:
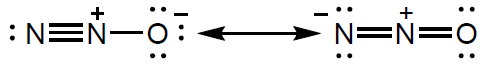
Outline why ozone in the stratosphere is important.
Dinitrogen monoxide in the stratosphere is converted to nitrogen monoxide, NO (g).
Write two equations to show how NO (g) catalyses the decomposition of ozone.
State one analytical technique that could be used to determine the ratio of 14N : 15N.
A sample of gas was enriched to contain 2 % by mass of 15N with the remainder being 14N.
Calculate the relative molecular mass of the resulting N2O.
Predict, giving two reasons, how the first ionization energy of 15N compares with that of 14N.
Explain why the first ionization energy of nitrogen is greater than both carbon and oxygen.
Nitrogen and carbon:
Nitrogen and oxygen:
State what the presence of alternative Lewis structures shows about the nature of the bonding in the molecule.
State, giving a reason, the shape of the dinitrogen monoxide molecule.
Deduce the hybridization of the central nitrogen atom in the molecule.
Carbon dioxide contributes significantly to global warming. It can be used as a raw material with methyloxirane to form polymers.
Suggest why the three-membered ring in methyloxirane is unstable.
Draw two structural isomers of methyloxirane.
State, giving a reason, whether methyloxirane can form cis-trans isomers.
Predict the chemical shift and splitting pattern of the signal produced by the hydrogen atoms labelled X in the 1H NMR spectrum of the polymer. Use section 27 of the data booklet.
Propene is an important starting material for many products. The following shows some compounds which can be made from propene, C3H6.
Propene (C3H6) → C3H7Cl → C3H8O → C3H6O
Consider the conversion of propene to C3H7Cl.
An experiment was carried out to determine the order of reaction between one of the isomers of C3H7Cl and aqueous sodium hydroxide. The following results were obtained.
State the type of reaction.
State the IUPAC name of the major product.
Outline why it is the major product.
Write an equation for the reaction of the major product with aqueous sodium hydroxide to produce a C3H8O compound, showing structural formulas.
Determine the rate expression from the results, explaining your method.
Deduce the type of mechanism for the reaction of this isomer of C3H7Cl with aqueous sodium hydroxide.
Sketch the mechanism using curly arrows to represent the movement of electrons.
Write an equation for the complete combustion of the compound C3H8O formed in (a)(iv).
Determine the enthalpy of combustion of this compound, in kJ mol−1, using data from section 11 of the data booklet.
State the reagents for the conversion of the compound C3H8O formed in (a)(iv) into C3H6O.
Explain why the compound C3H8O, produced in (a)(iv), has a higher boiling point than compound C3H6O, produced in d(i).
Explain why the 1H NMR spectrum of C3H6O, produced in (d)(i), shows only one signal.
Propene is often polymerized. Draw a section of the resulting polymer, showing two repeating units.
When heated in air, magnesium ribbon reacts with oxygen to form magnesium oxide.
The reaction in (a)(i) was carried out in a crucible with a lid and the following data was recorded:
Mass of crucible and lid = 47.372 ±0.001 g
Mass of crucible, lid and magnesium ribbon before heating = 53.726 ±0.001 g
Mass of crucible, lid and product after heating = 56.941 ±0.001 g
When magnesium is burnt in air, some of it reacts with nitrogen to form magnesium nitride according to the equation:
3 Mg (s) + N2 (g) → Mg3N2 (s)
The presence of magnesium nitride can be demonstrated by adding water to the product. It is hydrolysed to form magnesium hydroxide and ammonia.
Most nitride ions are 14N3–.
Write a balanced equation for the reaction that occurs.
Identify a metal, in the same period as magnesium, that does not form a basic oxide.
Calculate the amount of magnesium, in mol, that was used.
Determine the percentage uncertainty of the mass of product after heating.
Assume the reaction in (a)(i) is the only one occurring and it goes to completion, but some product has been lost from the crucible. Deduce the percentage yield of magnesium oxide in the crucible.
Evaluate whether this, rather than the loss of product, could explain the yield found in (b)(iii).
Suggest an explanation, other than product being lost from the crucible or reacting with nitrogen, that could explain the yield found in (b)(iii).
Calculate coefficients that balance the equation for the following reaction.
Ammonia is added to water that contains a few drops of an indicator. Identify an indicator that would change colour. Use sections 21 and 22 of the data booklet.
Determine the oxidation state of nitrogen in Mg3N2 and in NH3.
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
State the number of subatomic particles in this ion.
Some nitride ions are 15N3–. State the term that describes the relationship between 14N3– and 15N3–.
The nitride ion and the magnesium ion are isoelectronic (they have the same electron configuration). Determine, giving a reason, which has the greater ionic radius.
Suggest, giving a reason, whether magnesium or nitrogen would have the greater sixth ionization energy.
Suggest two reasons why atoms are no longer regarded as the indivisible units of matter.
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.