
SL Paper 2
Automobile air bags inflate by a rapid decomposition reaction. One typical compound used is guanidinium nitrate, C(NH2)3NO3, which decomposes very rapidly to form nitrogen, water vapour and carbon.
Deduce the equation for the decomposition of guanidinium nitrate.
Calculate the total number of moles of gas produced from the decomposition of 10.0 g of guanidinium nitrate.
Calculate the pressure, in kPa, of this gas in a 10.0 dm3 air bag at 127°C, assuming no gas escapes.
Suggest why water vapour deviates significantly from ideal behaviour when the gases are cooled, while nitrogen does not.
Another airbag reactant produces nitrogen gas and sodium.
Suggest, including an equation, why the products of this reactant present a safety hazard.
Markscheme
C(NH2)3NO3 (s) → 2N2 (g) + 3H2O (g) + C (s) ✔
moles of gas = « » 0.409 «mol» ✔
«» = 136 «kPa» ✔
Any two of:
nitrogen non-polar/London/dispersion forces AND water polar/H-bonding ✔
water has «much» stronger intermolecular forces ✔
water molecules attract/condense/occupy smaller volume «and therefore deviate from ideal behaviour» ✔
2Na (s) + 2H2O (l) → 2NaOH (aq) + H2 (g) ✔
hydrogen explosive
OR
highly exothermic reaction
OR
sodium reacts violently with water
OR
forms strong alkali ✔
NOTE: Accept the equation of combustion of hydrogen.
Do not accept just “sodium is reactive/dangerous”.
Examiners report
Explain the general increase in trend in the first ionization energies of the period 3 elements, Na to Ar.
Markscheme
increasing number of protons
OR
increasing nuclear charge ✔
«atomic» radius/size decreases
OR
same number of shells/electrons occupy same shell
OR
similar shielding «by inner electrons» ✔
Examiners report
There are many oxides of silver with the formula AgxOy. All of them decompose into their elements when heated strongly.
After heating 3.760 g of a silver oxide 3.275 g of silver remained. Determine the empirical formula of AgxOy.
Suggest why the final mass of solid obtained by heating 3.760 g of AgxOy may be greater than 3.275 g giving one design improvement for your proposed suggestion. Ignore any possible errors in the weighing procedure.
Naturally occurring silver is composed of two stable isotopes, 107Ag and 109Ag.
The relative atomic mass of silver is 107.87. Show that isotope 107Ag is more abundant.
Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each oxide was added to a separate 100 cm3 flask containing distilled water and a few drops of bromothymol blue indicator.
The indicator is listed in section 22 of the data booklet.
Deduce the colour of the resulting solution and the chemical formula of the product formed after reaction with water for each oxide.
Explain the electrical conductivity of molten Na2O and P4O10.
Outline the model of electron configuration deduced from the hydrogen line emission spectrum (Bohr’s model).
Markscheme
n(Ag) = «» 0.03036 «mol»
AND
n(O) = «» 0.03031 «mol»
« / ratio of Ag to O approximately 1 : 1, so»
AgO
Accept other valid methods for M1.
Award [1 max] for correct empirical formula if method not shown.
[2 marks]
temperature too low
OR
heating time too short
OR
oxide not decomposed completely
heat sample to constant mass «for three or more trials»
Accept “not heated strongly enough”.
If M1 as per markscheme, M2 can only be awarded for constant mass technique.
Accept "soot deposition" (M1) and any suitable way to reduce it (for M2).
Accept "absorbs moisture from atmosphere" (M1) and "cool in dessicator" (M2).
Award [1 max] for reference to impurity AND design improvement.
[2 marks]
Ar closer to 107/less than 108 «so more 107Ag»
OR
Ar less than the average of (107 + 109) «so more 107Ag»
Accept calculations that gives greater than 50% 107Ag.
[1 mark]
Do not accept name for the products.
Accept “Na+ + OH–” for NaOH.
Ignore coefficients in front of formula.
[3 marks]
«molten» Na2O has mobile ions/charged particles AND conducts electricity
«molten» P4O10 does not have mobile ions/charged particles AND does not conduct electricity/is poor conductor of electricity
Do not award marks without concept of mobile charges being present.
Award [1 max] if type of bonding or electrical conductivity correctly identified in each compound.
Do not accept answers based on electrons.
Award [1 max] if reference made to solution.
[2 marks]
electrons in discrete/specific/certain/different shells/energy levels
energy levels converge/get closer together at higher energies
OR
energy levels converge with distance from the nucleus
Accept appropriate diagram for M1, M2 or both.
Do not give marks for answers that refer to the lines in the spectrum.
[2 marks]
Examiners report
Magnesium is a group 2 metal which exists as a number of isotopes and forms many compounds.
State the nuclear symbol notation, , for magnesium-26.
Mass spectroscopic analysis of a sample of magnesium gave the following results:
Calculate the relative atomic mass, Ar, of this sample of magnesium to two decimal places.
Magnesium burns in air to form a white compound, magnesium oxide. Formulate an equation for the reaction of magnesium oxide with water.
Describe the trend in acid-base properties of the oxides of period 3, sodium to chlorine.
In addition to magnesium oxide, magnesium forms another compound when burned in air. Suggest the formula of this compound
Describe the structure and bonding in solid magnesium oxide.
Magnesium chloride can be electrolysed.
Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922 K and 987 K respectively.
Anode (positive electrode):
Cathode (negative electrode):
Markscheme
«Ar =»
«= 24.3269 =» 24.33
Award [2] for correct final answer.
Do not accept data booklet value (24.31).
MgO(s) + H2O(l) → Mg(OH)2(s)
OR
MgO(s) + H2O(l) → Mg2+(aq) + 2OH–(aq)
Accept .
from basic to acidic
through amphoteric
Accept “alkali/alkaline” for “basic”.
Accept “oxides of Na and Mg: basic AND oxide of Al: amphoteric” for M1.
Accept “oxides of non-metals/Si to Cl acidic” for M2.
Do not accept just “become more acidic”
Mg3N2
Accept MgO2, Mg(OH)2, Mg(NOx)2, MgCO3.
«3-D/giant» regularly repeating arrangement «of ions»
OR
lattice «of ions»
Accept “giant” for M1, unless “giant covalent” stated.
electrostatic attraction between oppositely charged ions
OR
electrostatic attraction between Mg2+ and O2– ions
Do not accept “ionic” without description.
Anode (positive electrode):
2Cl– → Cl2(g) + 2e–
Cathode (negative electrode):
Mg2+ + 2e– → Mg(l)
Penalize missing/incorrect state symbols at Cl2 and Mg once only.
Award [1 max] if equations are at wrong electrodes.
Accept Mg (g).
Examiners report
Iron may be extracted from iron (II) sulfide, FeS.
Iron (II) sulfide, FeS, is ionically bonded.
The first step in the extraction of iron from iron (II) sulfide is to roast it in air to form iron (III) oxide and sulfur dioxide.
Outline why metals, like iron, can conduct electricity.
Justify why sulfur is classified as a non-metal by giving two of its chemical properties.
Describe the bonding in this type of solid.
State the full electron configuration of the sulfide ion.
Outline, in terms of their electronic structures, why the ionic radius of the sulfide ion is greater than that of the oxide ion.
Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
Write the equation for this reaction.
Deduce the change in the oxidation state of sulfur.
Suggest why this process might raise environmental concerns.
Explain why the addition of small amounts of carbon to iron makes the metal harder.
Markscheme
mobile/delocalized «sea of» electrons
Any two of:
forms acidic oxides «rather than basic oxides» ✔
forms covalent/bonds compounds «with other non-metals» ✔
forms anions «rather than cations» ✔
behaves as an oxidizing agent «rather than a reducing agent» ✔
Award [1 max] for 2 correct non-chemical properties such as non-conductor, high ionisation energy, high electronegativity, low electron affinity if no marks for chemical properties are awarded.
electrostatic attraction ✔
between oppositely charged ions/between Fe2+ and S2− ✔
1s2 2s2 2p6 3s2 3p6 ✔
Do not accept “[Ne] 3s2 3p6”.
«valence» electrons further from nucleus/extra electron shell/ electrons in third/3s/3p level «not second/2s/2p»✔
Accept 2,8 (for O2–) and 2,8,8 (for S2–)
allows them to explain the properties of different compounds/substances
OR
enables them to generalise about substances
OR
enables them to make predictions ✔
Accept other valid answers.
4FeS(s) + 7O2(g) → 2Fe2O3(s) + 4SO2(g) ✔
Accept any correct ratio.
+6
OR
−2 to +4 ✔
Accept “6/VI”.
Accept “−II, 4//IV”.
Do not accept 2− to 4+.
sulfur dioxide/SO2 causes acid rain ✔
Accept sulfur dioxide/SO2/dust causes respiratory problems
Do not accept just “causes respiratory problems” or “causes acid rain”.
disrupts the regular arrangement «of iron atoms/ions»
OR
carbon different size «to iron atoms/ions» ✔
prevents layers/atoms sliding over each other ✔
Examiners report
Chlorine undergoes many reactions.
of manganese(IV) oxide was added to of .
Chlorine gas reacts with water to produce hypochlorous acid and hydrochloric acid.
is a common chlorofluorocarbon, .
State the full electron configuration of the chlorine atom.
State, giving a reason, whether the chlorine atom or the chloride ion has a larger radius.
Outline why the chlorine atom has a smaller atomic radius than the sulfur atom.
The mass spectrum of chlorine is shown.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Outline the reason for the two peaks at and .
Explain the presence and relative abundance of the peak at .
Calculate the amount, in , of manganese(IV) oxide added.
Determine the limiting reactant, showing your calculations.
Determine the excess amount, in , of the other reactant.
Calculate the volume of chlorine, in , produced if the reaction is conducted at standard temperature and pressure (STP). Use section 2 of the data booklet.
State the oxidation state of manganese in and .
Deduce, referring to oxidation states, whether is an oxidizing or reducing agent.
Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid.
State the formula of the conjugate base of hypochlorous acid.
Calculate the concentration of in a solution with a .
State the type of reaction occurring when ethane reacts with chlorine to produce chloroethane.
Predict, giving a reason, whether ethane or chloroethane is more reactive.
Write the equation for the reaction of chloroethane with a dilute aqueous solution of sodium hydroxide.
Deduce the nucleophile for the reaction in d(iii).
Ethoxyethane (diethyl ether) can be used as a solvent for this conversion. Draw the structural formula of ethoxyethane
Deduce the number of signals and their chemical shifts in the spectrum of ethoxyethane. Use section 27 of the data booklet.
Calculate the percentage by mass of chlorine in .
Comment on how international cooperation has contributed to the lowering of emissions responsible for ozone depletion.
Markscheme
✔
Do not accept condensed electron configuration.
AND more «electron–electron» repulsion ✔
Accept AND has an extra electron.
has a greater nuclear charge/number of protons/ «causing a stronger pull on the outer electrons» ✔
same number of shells
OR
same «outer» energy level
OR
similar shielding ✔
«two major» isotopes «of atomic mass and » ✔
«diatomic» molecule composed of «two» chlorine-37 atoms ✔
chlorine-37 is the least abundant «isotope»
OR
low probability of two «isotopes» occurring in a molecule ✔
✔
✔
AND is the limiting reactant ✔
Accept other valid methods of determining the limiting reactant in M2.
✔
✔
Accept methods employing .
✔
✔
oxidizing agent AND oxidation state of changes from to /decreases ✔
partially dissociates/ionizes «in water» ✔
✔
✔
«free radical» substitution/ ✔
Do not accept electrophilic or nucleophilic substitution.
chloroethane AND bond is weaker/ than bond/
OR
chloroethane AND contains a polar bond ✔
Accept “chloroethane AND polar”.
OR
✔
Accept use of and in the equation.
hydroxide «ion»/ ✔
Do not accept .
/ ✔
Accept .
«signals» ✔
AND ✔
Accept any values in the ranges.
Award [1 max] for two incorrect chemical shifts.
✔
✔
Award [2] for correct final answer.
Any of:
research «collaboration» for alternative technologies «to replace s»
OR
technologies «developed»/data could be shared
OR
political pressure/Montreal Protocol/governments passing legislations ✔
Do not accept just “collaboration”.
Do not accept any reference to as greenhouse gas or product of fossil fuel combustion.
Accept reference to specific measures, such as agreement on banning use/manufacture of s.
Examiners report
Most candidates wrote the electron configuration of chlorine correctly.
Only half of the candidates deduced that the chloride ion has a larger radius than the chlorine atom with a valid reason. Many candidates struggled with this question and decided that the extra electron in the chloride ion caused a greater attraction between the nucleus and the outer electrons.
Only about a third of the candidates identified the extra proton in the chlorine nucleus as the cause of the smaller atomic radius when compared to the sulfur atom, and only the stronger candidates also compared the shielding or the number of shells in the two atoms. Many candidates had a poor understanding of factors affecting atomic radius and could not explain the difference.
About 60% of the candidates recognized that the peaks at m/z 35 and 37 in the mass spectrum of chlorine are due to its isotopes. A few students wrote 'isomers' instead of 'isotopes'.
This was the lowest scoring question on the paper, that was also left blank by 10% of the candidates. About 20% of the candidates identified the peak at m/z = 74 to be due to a molecule made up of two 37Cl atoms. And only very few candidates commented that the low abundance of the peak was due to the low abundance of the 37Cl isotope. A common incorrect answer was that chlorine has an isotope of mass number 74.
Most candidates were able to determine the number of moles of MnO2 using the mass.
It was pleasing that the majority of the candidates were able to determine the limiting reactant by using the stoichiometric ratio.
Half of the candidates were able to determine the amount of excess reactant. Some candidates who determined the limiting reactant in the previous part correctly, forgot to use the stoichiometric ratio in this part, and ended up with incorrect answers.
60% of the candidates determined the volume of chlorine produced correctly. Some candidates made mistakes in the units when using PV = nRT and had a power of 10 error.
The majority of candidates were able to determine the oxidation states of Mn in the two compounds correctly.
Less than half of the candidates were awarded the mark. Some did identify MnO2 as the oxidizing agent but did not give the explanation in terms of oxidation state as required in the question. Other candidates did not have an understanding of oxidizing and reducing agents.
A very well answered question - 80% of candidates understood what is meant by the term weak acid. Incorrect answers included 'acids that have high pH'.
Half of the candidates deduced the formula of the conjugate base of hypochlorous acid. Incorrect answers included H2O and HCl.
A well answered question. It was pleasing to see that 70% of the candidates were able to calculate [H+] from the given pH.
More than half of the candidates identified the type of reaction between ethane and chlorine as a substitution reaction. A few candidates lost the marks for writing 'electrophilic substitution' or 'nucleophilic substitutions'.
This was a challenging question that was answered correctly by only 30% of the candidates. A variety of incorrect answers were seen such as 'chlorine is a halogen and hence it is reactive', and 'ethane is more reactive because it is an alkane'. For students who answered correctly, the polarity was the most frequently given reason.
Half of the candidates wrote the correct equation for the hydrolysis of chloroethane. Incorrect answers often included carbon dioxide and water as the products.
This was a highly discriminating question. Only 30% of the candidates were able to identify the hydroxide ion as the nucleophile in the hydrolysis of chloroethane. Incorrect answers included NaOH where the ion was not specified. 14% of the candidates left this question blank.
Half of the candidates were able to give the structural formula of ethoxyethane. Incorrect answers included methoxymethane, ketones and esters.
Nearly half of the candidates were able to identify the number of signals obtained in the 1H NMR spectrum of ethoxyethane, obtaining the first mark of this question. Many candidates were awarded the mark as 'error carried forward' from an incorrect structure of ethoxyethane. The second mark for this question required candidates to look up values of chemical shift from the data booklet. Nearly a third of the candidates were able to match the chemical environments of the hydrogen atoms in ethoxyethane to those listed in the data booklet successfully.
This was the highest scoring question in the paper. The majority of candidates were able to calculate the percentage by mass of chlorine in CCl2F2. Mistakes included incorrect rounding and arithmetic errors.
This nature of science question was well answered by half of the candidates. Some teachers commented that the wording was rather vague. Incorrect answers were mainly assuming that CFCs were related to the combustion of fuels and greenhouse gas emissions.
Calcium carbide, CaC2, is an ionic solid.
Describe the nature of ionic bonding.
State the electron configuration of the Ca2+ ion.
When calcium compounds are introduced into a gas flame a red colour is seen; sodium compounds give a yellow flame. Outline the source of the colours and why they are different.
Suggest two reasons why solid calcium has a greater density than solid potassium.
Outline why solid calcium is a good conductor of electricity.
Calcium carbide reacts with water to form ethyne and calcium hydroxide.
CaC2(s) + H2O(l) → C2H2(g) + Ca(OH)2(aq)
Estimate the pH of the resultant solution.
Markscheme
electrostatic attraction AND oppositely charged ions
[1 mark]
1s22s22p63s23p6
OR
[Ar]
[1 mark]
«promoted» electrons fall back to lower energy level
energy difference between levels is different
Accept “Na and Ca have different nuclear charge” for M2.
[2 marks]
Any two of:
stronger metallic bonding
smaller ionic/atomic radius
two electrons per atom are delocalized
OR
greater ionic charge
greater atomic mass
Do not accept just “heavier” or “more massive” without reference to atomic mass.
[2 marks]
delocalized/mobile electrons «free to move»
[1 mark]
pH > 7
Accept any specific pH value or range of values above 7 and below 14.
[1 mark]
Examiners report
Dinitrogen monoxide, N2O, causes depletion of ozone in the stratosphere.
Different sources of N2O have different ratios of 14N:15N.
Outline why ozone in the stratosphere is important.
State one analytical technique that could be used to determine the ratio of 14N:15N.
A sample of gas was enriched to contain 2 % by mass of 15N with the remainder being 14N.
Calculate the relative molecular mass of the resulting N2O.
Predict, giving two reasons, how the first ionization energy of 15N compares with that of 14N.
Suggest why it is surprising that dinitrogen monoxide dissolves in water to give a neutral solution.
Markscheme
absorbs UV/ultraviolet light «of longer wavelength than absorbed by O2» [✔]
mass spectrometry/MS [✔]
« » 14.02 [✔]
«Mr = (14.02 × 2) + 16.00 =» 44.04 [✔]
Any two:
same AND have same nuclear charge/number of protons/Zeff [✔]
same AND neutrons do not affect attraction/ionization energy/Zeff
OR
same AND neutrons have no charge [✔]
same AND same attraction for «outer» electrons [✔]
same AND have same electronic configuration/shielding [✔]
Note: Accept “almost the same”.
“same” only needs to be stated once.
oxides of nitrogen/non-metals are «usually» acidic [✔]
Examiners report
60 % of the candidates were aware that ozone in the atmosphere absorbs UV light. Some candidates did not gain the mark for not specifying the type of radiation absorbed.
Well answered. More than half of the candidates stated mass spectrometry is used to determine the ratio of the isotopes.
Many candidates successfully calculated the relative atomic mass of nitrogen in the sample. M2 was awarded independently of M1, so candidates who calculated the relative molecular mass using the Ar of nitrogen in the data booklet (14.01) were awarded M2. Many candidates scored both marks.
This was a challenging question for many candidates, while stronger candidates often showed clarity of thinking and were able to conclude that the ionization energies of the two isotopes must be the same and to provide two different reasons for this. Some candidates did realize that the ionization energies are similar but did not give the best reasons to support their answer. Many candidates thought the ionization energies would be different because the size of the nucleus was different. Some teachers commented that the question was difficult while others liked it because it made students apply their knowledge in an unfamiliar situation. The question had a good discrimination index.
Only a quarter of the candidates answered correctly. Some simply stated that N2O forms HNO3 with water which did not gain the mark.
The emission spectrum of an element can be used to identify it.
Elements show trends in their physical properties across the periodic table.
Draw the first four energy levels of a hydrogen atom on the axis, labelling n = 1, 2, 3 and 4.
Draw the lines, on your diagram, that represent the electron transitions to n = 2 in the emission spectrum.
Outline why atomic radius decreases across period 3, sodium to chlorine.
Outline why the ionic radius of K+ is smaller than that of Cl−.
Copper is widely used as an electrical conductor.
Draw arrows in the boxes to represent the electronic configuration of copper in the 4s and 3d orbitals.
Impure copper can be purified by electrolysis. In the electrolytic cell, impure copper is the anode (positive electrode), pure copper is the cathode (negative electrode) and the electrolyte is copper(II) sulfate solution.
Formulate the half-equation at each electrode.
Outline where and in which direction the electrons flow during electrolysis.
Markscheme
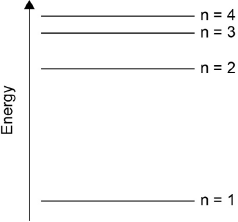
4 levels showing convergence at higher energy
[1 mark]
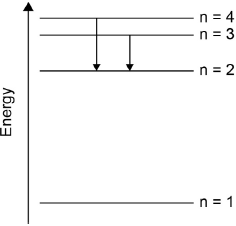
arrows (pointing down) from n = 3 to n = 2 AND n = 4 to n = 2
[1 mark]
same number of shells/«outer» energy level/shielding AND nuclear charge/number of protons/Zeff increases «causing a stronger pull on the outer electrons»
[1 mark]
K+ 19 protons AND Cl– 17 protons
OR
K+ has «two» more protons
same number of electrons/isoelectronic «thus pulled closer together»
[2 marks]
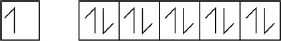
[1 mark]
Anode (positive electrode):
Cu(s) → Cu2+(aq) + 2e–
Cathode (negative electrode):
Cu2+(aq) + 2e– → Cu(s)
Accept Cu(s) – 2e– → Cu2+(aq).
Accept for →
Award [1 max] if the equations are at the wrong electrodes.
[2 marks]
«external» circuit/wire AND from positive/anode to negative/cathode electrode
Accept “through power supply/battery” instead of “circuit”.
[1 mark]
Examiners report
Electrons are arranged in energy levels around the nucleus of an atom.
The diagram represents possible electron energy levels in a hydrogen atom.
Explain why the first ionization energy of calcium is greater than that of potassium.
All models have limitations. Suggest two limitations to this model of the electron energy levels.
Draw an arrow, labelled X, to represent the electron transition for the ionization of a hydrogen atom in the ground state.
Draw an arrow, labelled Z, to represent the lowest energy electron transition in the visible spectrum.
Markscheme
increasing number of protons/nuclear charge/Zeff ✔
«atomic» radius/size decreases
OR
same number of energy levels
OR
similar shielding «by inner electrons» ✔
Any two of:
does not represent sub-levels/orbitals ✔
only applies to atoms with one electron/hydrogen ✔
does not explain why only certain energy levels are allowed ✔
the atom is considered to be isolated ✔
does not take into account the interactions between atoms/molecules/external fields ✔
does not consider the number of electrons the energy level can fit ✔
does not consider probability of finding electron at different positions/OWTTE ✔
Do not accept “does not represent distance «from nucleus»”.
upward arrow X AND starting at n = 1 extending to n = ∞ ✔
downward or upward arrow between n = 3 and n = 2 ✔
Examiners report
It was surprising that this question that appears regularly in IB chemistry papers was not better answered. Many candidates only obtained one of the two marks for identifying one factor (often the larger nuclear charge of calcium or that the number of shells was the same for Ca and K). However, a few candidates did write thorough answers reflecting a good understanding of the factors affecting ionization energy. This question had a strong correlation between candidates who scored well and those who had a high score overall. Some candidates did not score any marks by focusing on trends in the Periodic Table without offering an explanation, or by discussing the number of electrons in Ca and K instead of the number of protons.
Only 30% of the candidates drew the correct arrow on the diagram representing the ionization of hydrogen. A few candidates missed the mark by having the arrow pointing downwards. The most common incorrect answer was a transition between n=1 and n=2.
Rhenium, Re, was the last element with a stable isotope to be isolated.
One chloride of rhenium has the empirical formula ReCl3.
Before its isolation, scientists predicted the existence of rhenium and some of its properties.
Suggest the basis of these predictions.
Describe how the relative reactivity of rhenium, compared to silver, zinc, and copper, can be established using pieces of rhenium and solutions of these metal sulfates.
State the name of this compound, applying IUPAC rules.
Calculate the percentage, by mass, of rhenium in ReCl3.
Markscheme
gap in the periodic table
OR
element with atomic number «75» unknown
OR
break/irregularity in periodic trends [✔]
«periodic table shows» regular/periodic trends «in properties» [✔]
place «pieces of» Re into each solution [✔]
if Re reacts/is coated with metal, that metal is less reactive «than Re» [✔]
Note: Accept other valid observations such as “colour of solution fades” or “solid/metal appears” for “reacts”.
rhenium(III) chloride
OR
rhenium trichloride [✔]
«Mr ReCl3 = 186.21 + (3 × 35.45) =» 292.56 [✔]
«100 × =» 63.648 «%» [✔]
Examiners report
This nature of science question generated a lot of discussion among teachers. Some in support of such questions and others concerned that it takes a lot of time for candidates to know how to answer. Some teachers thought it was unclear what the question was asking. It is pleasing that about a quarter of the candidates answered both parts successfully and many candidates gained one mark usually for “periodic trends”. However, some candidates only focused on one part of the question. Quite a few candidates discussed isotopes, probably thrown off by the stem. A teacher was concerned that since transition metals are not part of the SL syllabus that Re was a bad choice, however, the question did not really require any transition metal chemistry to be answered.
This question was a good discriminator between high-scoring and low-scoring candidates. It was well answered by more than half of the candidates who had obviously carried out such displacement reactions and interpreted the outcomes during the course. Some candidates did not state the obvious of dipping the metal into the sulfates.
More than half of the candidates named ReCl3 correctly. Common mistakes included “rhenium chloride” and “trichlororhenium”.
The majority of candidates calculated the percentage, by mass, of rhenium in ReCl3 correctly. Some rounding errors were seen that students should be more careful with.
Trends in physical and chemical properties are useful to chemists.
The Activity series lists the metal in order of reactivity.
Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
State an equation for the reaction of phosphorus (V) oxide, P4O10 (s), with water.
Describe the emission spectrum of hydrogen.
Identify the strongest reducing agent in the given list.
A voltaic cell is made up of a Mn2+/Mn half-cell and a Ni2+/Ni half-cell.
Deduce the equation for the cell reaction.
The voltaic cell stated in part (ii) is partially shown below.
Draw and label the connections needed to show the direction of electron movement and ion flow between the two half-cells.
Markscheme
increasing number of protons
OR
increasing nuclear charge
«atomic» radius/size decreases
OR
same number of shells
OR
similar shielding «by inner electrons»
«greater energy needed to overcome increased attraction between nucleus and electrons»
atomic/ionic radius increases
smaller charge density
OR
force of attraction between metal ions and delocalised electrons decreases
Do not accept discussion of attraction between valence electrons and
nucleus for M2.
Accept “weaker metallic bonds” for M2.
P4O10 (s) + 6H2O (l) → 4H3PO4 (aq)
Accept “P4O10 (s) + 2H2O (l) → 4HPO3 (aq)” (initial reaction).
«series of» lines
OR
only certain frequencies/wavelengths
convergence at high«er» frequency/energy/short«er» wavelength
M1 and/or M2 may be shown on a diagram.
Mn
Mn (s) + Ni2+ (aq) → Ni (s) + Mn2+ (aq)
wire between electrodes AND labelled salt bridge in contact with both electrolytes
anions to right (salt bridge)
OR
cations to left (salt bridge)
OR
arrow from Mn to Ni (on wire or next to it)
Electrodes can be connected directly or through voltmeter/ammeter/light bulb, but not a battery/power supply.
Accept ions or a specific salt as the label of the salt bridge.
Examiners report
Magnesium is a reactive metal often found in alloys.
Organomagnesium compounds can react with carbonyl compounds. One overall equation is:
Compound B can also be prepared by reacting an alkene with water.
Iodomethane is used to prepare CH3Mg. It can also be converted into methanol:
CH3 + HO– → CH3OH + –
Magnesium can be produced by the electrolysis of molten magnesium chloride.
Write the half-equation for the formation of magnesium.
Suggest an experiment that shows that magnesium is more reactive than zinc, giving the observation that would confirm this.
State the name of Compound A, applying International Union of Pure and Applied Chemistry (IUPAC) rules.
Identify the strongest force between the molecules of Compound B.
Draw the structural formula of the alkene required.
Deduce the structural formula of the repeating unit of the polymer formed from this alkene.
Deduce what would be observed when Compound B is warmed with acidified aqueous potassium dichromate (VI).
Identify the type of reaction.
Outline the requirements for a collision between reactants to yield products.
The polarity of the carbon–halogen bond, C–X, facilitates attack by HO–.
Outline, giving a reason, how the bond polarity changes going down group 17.
Markscheme
Mg2+ + 2 e- → Mg ✔
Do not penalize missing charge on electron.
Accept equation with equilibrium arrows.
Alternative 1
put Mg in Zn2+(aq) ✔
Zn/«black» layer forms «on surface of Mg» ✔
Award [1 max] for “no reaction when Zn placed in Mg2+(aq)”.
Alternative 2
place both metals in acid ✔
bubbles evolve more rapidly from Mg
OR
Mg dissolves faster ✔
Alternative 3
construct a cell with Mg and Zn electrodes ✔
bulb lights up
OR
shows (+) voltage
OR
size/mass of Mg(s) decreases «over time»
OR
size/mass of Zn increases «over time»
Accept “electrons flow from Mg to Zn”.
Accept Mg is negative electrode/anode
OR
Zn is positive electrode/cathode
Accept other correct methods.
propanone ✔
Accept 2-propanone and propan-2-one.
hydrogen bonds ✔
Do not penalize missing brackets or n.
Do not award mark if continuation bonds are not shown.
no change «in colour/appearance/solution» ✔
«nucleophilic» substitution
OR
SN2 ✔
Accept “hydrolysis”.
Accept SN1
energy/E ≥ activation energy/Ea ✔
correct orientation «of reacting particles»
OR
correct geometry «of reacting particles» ✔
decreases/less polar AND electronegativity «of the halogen» decreases ✔
Accept “decreases” AND a correct comparison of the electronegativity of two halogens.
Accept “decreases” AND “attraction for valence electrons decreases”.
Examiners report
Unfortunately, only 40% of the students could write this quite straightforward half equation.
Many candidates gained some credit by suggesting voltaic cell or a displacement reaction, but most could not gain the second mark and the reason was often a failure to be able to differentiate between "what occurs" and "what is observed".
Even though superfluous numbers (2-propanone, propan-2-one) were overlooked, only about half of the students could correctly name this simple molecule.
Probably just over half the students correctly identified hydrogen bonding, with dipole-dipole being the most common wrong answer, though a significant number identified an intramolecular bond.
Few candidates could correctly eliminate water to deduce the identity of the required reactant.
Correct answers to this were very scarce and even when candidates had an incorrect alkene for the previous part, they were unable to score an ECF mark, by deducing the formula of the polymer it would produce.
Some students deduced that, as it was a tertiary alcohol, there would be no reaction, but almost all were lucky that this was accepted as well as the correct observation - "it would remain orange".
About a quarter of the students identified this as a substitution reaction, though quite a number then lost the mark by incorrectly stating it was either "free radical" or "electrophilic". A very common wrong answer was "displacement" or "single displacement" and this makes one wonder whether this terminology is being taught instead of substitution
Generally well done with the vast majority of students correctly citing "correct orientation" and many only failed to gain the second mark through failing to equate the energy required to the activation energy.
Another question that was not well answered with probably only a quarter of candidates stating that the polarity would decrease because of decreasing electronegativity down the group.
The properties of elements can be predicted from their position in the periodic table.
Explain why Si has a smaller atomic radius than Al.
Explain the decrease in radius from Na to Na+.
State the condensed electron configurations for Cr and Cr3+.
Describe metallic bonding and how it contributes to electrical conductivity.
Deduce the Lewis (electron dot) structure and molecular geometry of sulfur dichloride, SCl2.
Suggest, giving reasons, the relative volatilities of SCl2 and H2O.
Consider the following equilibrium reaction:
2SO2 (g) + O2 (g) 2SO3 (g)
State and explain how the equilibrium would be affected by increasing the volume of the reaction container at a constant temperature.
Markscheme
nuclear charge/number of protons/Z/Zeff increases «causing a stronger pull on the outer electrons» ✓
same number of shells/«outer» energy level/shielding ✓
Na+ has one less energy level/shell
OR
Na+ has 2 energy levels/shells AND Na has 3 ✓
less shielding «in Na+ so valence electrons attracted more strongly to nucleus»
OR
effective nuclear charge/Zeff greater «in Na+ so valence electrons attracted more strongly to nucleus» ✓
Accept “more protons than electrons «in Na+»” OR “less electron-electron repulsion «in Na+»” for M2.
Cr:
[Ar] 4s13d5 ✓
Cr3+:
[Ar] 3d3 ✓
Accept “[Ar] 3d54s1”.
Accept “[Ar] 3d34s0”.
Award [1 max] for two correct full electron configurations “1s22s22p63s23p64s13d5 AND 1s22s22p63s23p63d3”.
Award [1 max] for 4s13d5 AND 3d3.
electrostatic attraction ✓
between «a lattice of» cations/positive «metal» ions AND «a sea of» delocalized electrons ✓
mobile electrons responsible for conductivity
OR
electrons move when a voltage/potential difference/electric field is applied ✓
Do not accept “nuclei” for “cations/positive ions” in M2.
Accept “mobile/free” for “delocalized” electrons in M2.
Accept “electrons move when connected to a cell/battery/power supply” OR “electrons move when connected in a circuit” for M3.
H2O forms hydrogen bonding «while SCl2 does not» ✓
SCl2 «much» stronger London/dispersion/«instantaneous» induced dipole-induced dipole forces ✓
Alternative 1:
H2O less volatile AND hydrogen bonding stronger «than dipole–dipole and dispersion forces» ✓
Alternative 2:
SCl2 less volatile AND effect of dispersion forces «could be» greater than hydrogen bonding ✓\
Ignore reference to Van der Waals.
Accept “SCl2 has «much» larger molar mass/electron density” for M2.
pressure decrease «due to larger volume» ✓
reactant side has more moles/molecules «of gas» ✓
reaction shifts left/towards reactants ✓
Award M3 only if M1 OR M2 is awarded.
Examiners report
Properties of elements and their compounds can be related to the position of the elements in the periodic table.
Explain the decrease in atomic radius from Na to Cl.
Explain why the radius of the sodium ion, Na+, is smaller than the radius of the oxide ion, O2−.
State a physical property of sodium oxide.
Markscheme
nuclear charge/number of protons/Zeff increases «causing a stronger pull on the outer electrons» ✔
same number of shells/«outer» energy level/shielding ✔
Accept “atomic number” for “number of protons”.
isoelectronic/same electronic configuration/«both» have 2.8 ✔
more protons in Na+ ✔
Any one of:
brittle ✔
high melting point/crystalline/solid «at room temperature» ✔
low volatility ✔
conducts electricity when molten ✔
does not conduct electricity at room temperature ✔
Do not accept soluble in water.
Ignore any chemical properties.
Examiners report
Lithium reacts with water to form an alkaline solution.
A 0.200 g piece of lithium was placed in 500.0 cm3 of water.
Determine the coefficients that balance the equation for the reaction of lithium with water.
Calculate the molar concentration of the resulting solution of lithium hydroxide.
Calculate the volume of hydrogen gas produced, in cm3, if the temperature was 22.5 °C and the pressure was 103 kPa. Use sections 1 and 2 of the data booklet.
Suggest a reason why the volume of hydrogen gas collected was smaller than predicted.
The reaction of lithium with water is a redox reaction. Identify the oxidizing agent in the reaction giving a reason.
Describe two observations that indicate the reaction of lithium with water is exothermic.
Markscheme
2 Li (s) + 2 H2O (l) → 2 LiOH (aq) + H2 (g) ✔
✔
«nLiOH = nLi»
✔
Award [2] for correct final answer.
✔
✔
Award [2] for correct final answer.
Accept answers in the range 334 – 344 cm3.
Award [1 max] for 0.343 «cm3/dm3/m3».
Award [1 max] for 26.1 cm3 obtained by using 22.5 K.
Award [1 max] for 687 cm3 obtained by using 0.0288 mol.
lithium was impure/«partially» oxidized
OR
gas leaked/ignited ✔
Accept “gas dissolved”.
H2O AND hydrogen gains electrons «to form H2»
OR
H2O AND H oxidation state changed from +1 to 0 ✔
Accept “H2O AND H/H2O is reduced”.
Any two:
temperature of the water increases ✔
lithium melts ✔
pop sound is heard ✔
Accept “lithium/hydrogen catches fire”.
Do not accept “smoke is observed”.
Examiners report
This part-question was better answered than part (ii). 50% of the candidates drew a correct arrow between n=2 and n=3. Both absorption and emission transitions were accepted since the question did not specify which type of spectrum was required. Some teachers commented on this in their feedback. Mistakes often included transitions between higher energy levels.
This question is about compounds of sodium.
Sodium peroxide is used in diving apparatus to produce oxygen from carbon dioxide.
2Na2O2 (s) + 2CO2 (g) → 2Na2CO3 (s) + O2 (g)
Describe the structure and bonding in solid sodium oxide.
Write equations for the separate reactions of solid sodium oxide and solid phosphorus(V) oxide with excess water and differentiate between the solutions formed.
Sodium oxide, Na2O:
Phosphorus(V) oxide, P4O10:
Differentiation:
Sodium peroxide, Na2O2, is formed by the reaction of sodium oxide with oxygen.
2Na2O (s) + O2 (g) → 2Na2O2 (s)
Calculate the percentage yield of sodium peroxide if 5.00 g of sodium oxide produces 5.50 g of sodium peroxide.
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
Outline why bond enthalpy values are not valid in calculations such as that in (c)(i).
The reaction of sodium peroxide with excess water produces hydrogen peroxide and one other sodium compound. Suggest the formula of this compound.
State the oxidation number of carbon in sodium carbonate, Na2CO3.
Markscheme
«3-D/giant» regularly repeating arrangement «of ions»
OR
lattice «of ions» [✔]
electrostatic attraction between oppositely charged ions
OR
electrostatic attraction between Na+ and O2− ions [✔]
Note: Do not accept “ionic” without description.
Sodium oxide:
Na2O(s) + H2O(l) → 2NaOH (aq) [✔]
Phosphorus(V) oxide:
P4O10 (s) + 6H2O(l) → 4H3PO4 (aq) [✔]
Differentiation:
NaOH / product of Na2O is alkaline/basic/pH > 7 AND H3PO4 / product of P4O10 is acidic/pH < 7 [✔]
n(Na2O2) theoretical yield «= » = 0.0807/8.07 × 10−2 «mol»
OR
mass Na2O2 theoretical yield «= × 77.98 gmol−1» = 6.291 «g» [✔]
% yield «= × 100» OR « x 100» = 87.4 «%» [✔]
Note: Award [2] for correct final answer.
ΣΔHf products = 2 × (−1130.7) / −2261.4 «kJ» [✔]
ΣΔHf reactants = 2 × (−510.9) + 2 × (−393.5) / −1808.8 «kJ» [✔]
ΔH = «ΣΔHf products − ΣΔHf reactants = −2261.4 −(−1808.8) =» −452.6 «kJ» [✔]
Note: Award [3] for correct final answer.
Award [2 max] for “+452.6 «kJ»”.
only valid for covalent bonds
OR
only valid in gaseous state [✔]
NaOH [✔]
Note: Accept correct equation showing NaOH as a product.
IV [✔]
Examiners report
Disappointingly many students did not realise that sodium oxide was held by ionic bonds, many said it was covalent or metallic bonding. The ones that knew it was ionic failed to describe it adequately to earn the 2 marks.
Very few students could correctly write out the two equations and so often were unable to realise it was acid/base behaviour that would differentiate the oxides.
Many candidates were able to correctly calculate the % yield but some weaker candidates just used 5.0/5.5 to find %.
The calculation of the enthalpy change using enthalpies of formation was generally answered well but common mistakes were students forgetting to multiply by 2 or adding extra terms for oxygen.
Most students didn’t gain a mark and “values are average” was the most common incorrect answer. The fact this was an ionic compound did not register with them. Some students did gain a mark for stating that the substances were not in a gaseous state.
Some students correctly identified sodium hydroxide as the correct product, but hydrogen, oxygen and sodium oxide were common answers.
Oxidation number of +4 was often correctly identified.
When heated in air, magnesium ribbon reacts with oxygen to form magnesium oxide.
The reaction in (a)(i) was carried out in a crucible with a lid and the following data was recorded:
Mass of crucible and lid = 47.372 ±0.001 g
Mass of crucible, lid and magnesium ribbon before heating = 53.726 ±0.001 g
Mass of crucible, lid and product after heating = 56.941 ±0.001 g
When magnesium is burnt in air, some of it reacts with nitrogen to form magnesium nitride according to the equation:
3 Mg (s) + N2 (g) → Mg3N2 (s)
The presence of magnesium nitride can be demonstrated by adding water to the product. It is hydrolysed to form magnesium hydroxide and ammonia.
Most nitride ions are 14N3–.
Write a balanced equation for the reaction that occurs.
State the block of the periodic table in which magnesium is located.
Identify a metal, in the same period as magnesium, that does not form a basic oxide.
Calculate the amount of magnesium, in mol, that was used.
Determine the percentage uncertainty of the mass of product after heating.
Assume the reaction in (a)(i) is the only one occurring and it goes to completion, but some product has been lost from the crucible. Deduce the percentage yield of magnesium oxide in the crucible.
Evaluate whether this, rather than the loss of product, could explain the yield found in (b)(iii).
Suggest an explanation, other than product being lost from the crucible or reacting with nitrogen, that could explain the yield found in (b)(iii).
Calculate coefficients that balance the equation for the following reaction.
__ Mg3N2 (s) + __ H2O (l) → __ Mg(OH)2 (s) + __ NH3 (aq)
Determine the oxidation state of nitrogen in Mg3N2 and in NH3.
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
State the number of subatomic particles in this ion.
Some nitride ions are 15N3–. State the term that describes the relationship between 14N3– and 15N3–.
The nitride ion and the magnesium ion are isoelectronic (they have the same electron configuration). Determine, giving a reason, which has the greater ionic radius.
Suggest two reasons why atoms are no longer regarded as the indivisible units of matter.
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.
Markscheme
2 Mg(s) + O2(g) → 2 MgO(s) ✔
Do not accept equilibrium arrows. Ignore state symbols
s ✔
Do not allow group 2
aluminium/Al ✔
«mol» ✔
mass of product ✔
✔
Award [2] for correct final answer
Accept 0.021%
✔
✔
Award «0.2614 mol x 40.31 g mol–1»
Accept alternative methods to arrive at the correct answer.
Accept final answers in the range 91-92%
[2] for correct final answer.
yes
AND
«each Mg combines with N, so» mass increase would be 14x which is less than expected increase of 16x
OR
3 mol Mg would form 101g of Mg3N2 but would form 3 x MgO = 121 g of MgO
OR
0.2614 mol forms 10.536 g of MgO, but would form 8.796 g of Mg3N2 ✔
Accept Yes AND “the mass of N/N2 that combines with each g/mole of Mg is lower than that of O/O2”
Accept YES AND “molar mass of nitrogen less than of oxygen”.
incomplete reaction
OR
Mg was partially oxidised already
OR
impurity present that evaporated/did not react ✔
Accept “crucible weighed before fully cooled”.
Accept answers relating to a higher atomic mass impurity consuming less O/O2.
Accept “non-stoichiometric compounds formed”.
Do not accept "human error", "wrongly calibrated balance" or other non-chemical reasons.
If answer to (b)(iii) is >100%, accept appropriate reasons, such as product absorbed moisture before being weighed.
«1» Mg3N2 (s) + 6 H2O (l) → 3 Mg(OH)2 (s) + 2 NH3 (aq)
Mg3N2: -3
AND
NH3: -3 ✔
Do not accept 3 or 3-
Acid–base:
yes AND N3- accepts H+/donates electron pair«s»
OR
yes AND H2O loses H+ «to form OH-»/accepts electron pair«s» ✔
Redox:
no AND no oxidation states change ✔
Accept “yes AND proton transfer takes place”
Accept reference to the oxidation state of specific elements not changing.
Accept “not redox as no electrons gained/lost”.
Award [1 max] for Acid–base: yes AND Redox: no without correct reasons, if no other mark has been awarded
Protons: 7 AND Neutrons: 7 AND Electrons: 10 ✔
isotope«s» ✔
nitride AND smaller nuclear charge/number of protons/atomic number ✔
Any two of:
subatomic particles «discovered»
OR
particles smaller/with masses less than atoms «discovered»
OR
«existence of» isotopes «same number of protons, different number of neutrons» ✔
charged particles obtained from «neutral» atoms
OR
atoms can gain or lose electrons «and become charged» ✔
atom «discovered» to have structure ✔
fission
OR
atoms can be split ✔
Accept atoms can undergo fusion «to produce heavier atoms»
Accept specific examples of particles.
Award [2] for “atom shown to have a nucleus with electrons around it” as both M1 and M3.
Award [1] for all bonding types correct.
Award [1] for each correct description.
Apply ECF for M2 only once.
Examiners report
This was not as well done as one might have expected with the most common errors being O instead of O2 oxygen and MgO rather than MgO2.
Many students did not know what "block" meant, and often guessed group 2 etc.
Many students confused "period" and "group" and also many did not read metal, so aluminium was not chosen by the majority.
A number of students were not able to interpret the results and hence find the gain in mass and calculate the moles correctly.
Only a handful could work out the correct answer. Most had no real idea and quite a lot of blank responses. There also seems to be significant confusion between "percent uncertainty" and "percent error".
This was not well answered, but definitely better than the previous question with quite a few gaining some credit for correctly determining the theoretical yield.
This proved to be a very difficult question to answer in the quantitative manner required, with hardly any correct responses.
Quite a few students realised that incomplete reaction would lead to this, but only 30% of students gave a correct answer rather than a non-specific guess, such as "misread balance" or "impurities".
This was generally very well done with almost all candidates being able to determine the correct coefficients.
About 40% of students managed to correctly determine both the oxidation states, as -3, with errors being about equally divided between the two compounds.
Probably only about 10% could explain why this was an acid-base reaction. Rather more made valid deductions about redox, based on their answer to the previous question.
Most candidates could answer the question about subatomic particles correctly.
Identification of isotopes was answered correctly by most students.
In spite of being given the meaning of "isoelectronic", many candidates talked about the differing number of electrons and only about 30% could correctly analyse the situation in terms of nuclear charge.
The question was marked quite leniently so that the majority of candidates gained at least one of the marks by mentioning a subatomic particle. A significant number read "indivisible" as "invisible" however.
About a quarter of the students gained full marks and probably a similar number gained no marks. Metallic bonding was the type that seemed least easily recognised and least easily described. Another common error was to explain ionic bonding in terms of attraction of ions rather than describing electron transfer.
Titanium is a transition metal.
TiCl4 reacts with water and the resulting titanium(IV) oxide can be used as a smoke screen.
Describe the bonding in metals.
Titanium exists as several isotopes. The mass spectrum of a sample of titanium gave the following data:
Calculate the relative atomic mass of titanium to two decimal places.
State the number of protons, neutrons and electrons in the atom.
State the full electron configuration of the 2+ ion.
Explain why an aluminium-titanium alloy is harder than pure aluminium.
State the type of bonding in potassium chloride which melts at 1043 K.
A chloride of titanium, TiCl4, melts at 248 K. Suggest why the melting point is so much lower than that of KCl.
Formulate an equation for this reaction.
Suggest one disadvantage of using this smoke in an enclosed space.
Markscheme
electrostatic attraction
between «a lattice of» metal/positive ions/cations AND «a sea of» delocalized electrons
Accept mobile electrons.
Do not accept “metal atoms/nuclei”.
[2 marks]
= 47.93
Answer must have two decimal places with a value from 47.90 to 48.00.
Award [2] for correct final answer.
Award [0] for 47.87 (data booklet value).
[2 marks]
Protons: 22 AND Neutrons: 26 AND Electrons: 22
[1 mark]
1s22s22p63s23p63d2
[1 mark]
titanium atoms/ions distort the regular arrangement of atoms/ions
OR
titanium atoms/ions are a different size to aluminium «atoms/ions»
prevent layers sliding over each other
Accept diagram showing different sizes of atoms/ions.
[2 marks]
ionic
OR
«electrostatic» attraction between oppositely charged ions
[1 mark]
«simple» molecular structure
OR
weak«er» intermolecular bonds
OR
weak«er» bonds between molecules
Accept specific examples of weak bonds such as London/dispersion and van der Waals.
Do not accept “covalent”.
[1 mark]
TiCl4(l) + 2H2O(l) → TiO2(s) + 4HCl(aq)
correct products
correct balancing
Accept ionic equation.
Award M2 if products are HCl and a compound of Ti and O.
[2 marks]
HCl causes breathing/respiratory problems
OR
HCl is an irritant
OR
HCl is toxic
OR
HCl has acidic vapour
OR
HCl is corrosive
Accept “TiO2 causes breathing problems/is an irritant”.
Accept “harmful” for both HCl and TiO2.
Accept “smoke is asphyxiant”.
[1 mark]