
HL Paper 2
The equations show steps in the formation and decomposition of ozone in the stratosphere, some of which absorb ultraviolet light.
Step 1 O2 → 2O•
Step 2 O• + O2 → O3
Step 3 O3 → O• + O2
Step 4 O• + O3 → 2O2
Draw the Lewis structures of oxygen, O2, and ozone, O3.
Outline why both bonds in the ozone molecule are the same length and predict the bond length in the ozone molecule. Refer to section 10 of the data booklet.
Reason:
Length:
Predict the bond angle in the ozone molecule.
Discuss how the different bond strengths between the oxygen atoms in O2 and O3 in the ozone layer affect radiation reaching the Earth’s surface.
Identify the steps which absorb ultraviolet light.
Determine, showing your working, the wavelength, in m, of ultraviolet light absorbed by a single molecule in one of these steps. Use sections 1, 2 and 11 of the data booklet.
Ozone depletion is catalysed by nitrogen monoxide, NO, which is produced in aircraft and motor vehicle engines, and has the following Lewis structure.
Show how nitrogen monoxide catalyses the decomposition of ozone, including equations in your answer.
Magnesium is a reactive metal often found in alloys.
Magnesium is sometimes used as a sacrificial anode to protect steel from corrosion.
A graph of the volume of gas produced by reacting magnesium with a large excess of 1 mol dm–3 hydrochloric acid is shown.
Suggest an experiment that shows that magnesium is more reactive than zinc, giving the observation that would confirm this.
Calculate the standard potential, in V, of a cell formed by magnesium and steel half-cells. Use section 24 of the data booklet and assume steel has the standard electrode potential of iron.
Calculate the free energy change, ΔG⦵, in kJ, of the cell reaction. Use sections 1 and 2 of the data booklet.
This cell causes the electrolytic reduction of water on the steel. State the half-equation for this reduction.
Use the graph to deduce the dependence of the reaction rate on the amount of Mg.
The reaction is first order with respect to HCl. Calculate the time taken, in seconds (s), for half of the Mg to dissolve when [HCl] = 0.5 mol dm–3.
Carbonates also react with HCl and the rate can be determined by graphing the mass loss. Suggest why this method is less suitable for the reaction of Mg with HCl.
The rate of the acid-catalysed iodination of propanone can be followed by measuring how the concentration of iodine changes with time.
I2(aq) + CH3COCH3(aq) → CH3COCH2I(aq) + H+(aq) + I−(aq)
The general form of the rate equation is:
Rate = [H3CCOCH3(aq)]m × [I2(aq)]n × [H+(aq)]p
The reaction is first order with respect to propanone.
Suggest how the change of iodine concentration could be followed.
A student produced these results with . Propanone and acid were in excess and iodine was the limiting reagent. Determine the relative rate of reaction when .
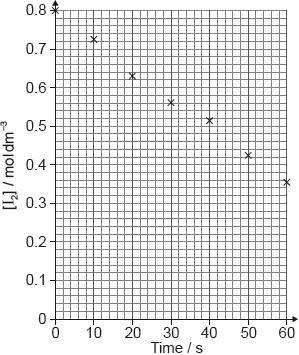
The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
Determine the relationship between the rate of reaction and the concentration of acid and the order of reaction with respect to hydrogen ions.
When the concentration of iodine is varied, while keeping the concentrations of acid and propanone constant, the following graphs are obtained.
Deduce, giving your reason, the order of reaction with respect to iodine.
When the reaction is carried out in the absence of acid the following graph is obtained.
Discuss the shape of the graph between A and B.
Sodium thiosulfate solution reacts with dilute hydrochloric acid to form a precipitate of sulfur at room temperature.
Na2S2O3 (aq) + 2HCl (aq) → S (s) + SO2 (g) + 2NaCl (aq) + X
(i) Using the graph, explain the order of reaction with respect to sodium thiosulfate.
(ii) In a different experiment, this reaction was found to be first order with respect to hydrochloric acid. Deduce the overall rate expression for the reaction.
Calcium carbonate reacts with hydrochloric acid.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
The results of a series of experiments in which the concentration of HCl was varied are shown below.
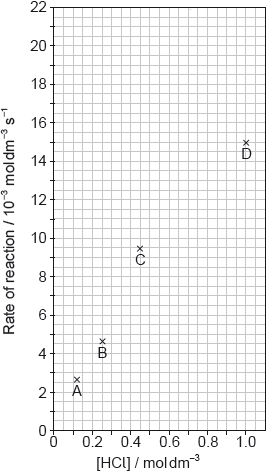
Outline two ways in which the progress of the reaction can be monitored. No practical details are required.
Suggest why point D is so far out of line assuming human error is not the cause.
Draw the best fit line for the reaction excluding point D.
Suggest the relationship that points A, B and C show between the concentration of the acid and the rate of reaction.
Deduce the rate expression for the reaction.
Calculate the rate constant of the reaction, stating its units.
Predict from your line of best fit the rate of reaction when the concentration of HCl is 1.00 mol dm−3.
Describe how the activation energy of this reaction could be determined.
The thermal decomposition of dinitrogen monoxide occurs according to the equation:
2N2O (g) → 2N2 (g) + O2 (g)
The reaction can be followed by measuring the change in total pressure, at constant temperature, with time.
The x-axis and y-axis are shown with arbitrary units.
This decomposition obeys the rate expression:
= k[N2O]
Explain why, as the reaction proceeds, the pressure increases by the amount shown.
Outline, in terms of collision theory, how a decrease in pressure would affect the rate of reaction.
Deduce how the rate of reaction at t = 2 would compare to the initial rate.
It has been suggested that the reaction occurs as a two-step process:
Step 1: N2O (g) → N2 (g) + O (g)
Step 2: N2O (g) + O (g) → N2 (g) + O2 (g)
Explain how this could support the observed rate expression.
The experiment is repeated using the same amount of dinitrogen monoxide in the same apparatus, but at a lower temperature.
Sketch, on the axes in question 2, the graph that you would expect.
The experiment gave an error in the rate because the pressure gauge was inaccurate.
Outline whether repeating the experiment, using the same apparatus, and averaging the results would reduce the error.
The graph below shows the Maxwell–Boltzmann distribution of molecular energies at a particular temperature.
The rate at which dinitrogen monoxide decomposes is significantly increased by a metal oxide catalyst.
Annotate and use the graph to outline why a catalyst has this effect.
Determine the standard entropy change, in J K−1, for the decomposition of dinitrogen monoxide.
2N2O (g) → 2N2 (g) + O2 (g)
Dinitrogen monoxide has a positive standard enthalpy of formation, ΔHfθ.
Deduce, giving reasons, whether altering the temperature would change the spontaneity of the decomposition reaction.
Hydrogen and iodine react to form hydrogen iodide.
H2 (g) + 2 (g) 2H (g)
The following experimental data was obtained.
Consider the reaction of hydrogen with solid iodine.
H2 (g) + 2 (s) 2H (g) ΔH⦵ = +53.0 kJ mol−1
Deduce the order of reaction with respect to hydrogen.
Deduce the rate expression for the reaction.
Calculate the value of the rate constant stating its units.
State two conditions necessary for a successful collision between reactants.
State the equilibrium constant expression, Kc, for this reaction.
Calculate the entropy change of reaction, ΔS⦵, in J K−1 mol−1.
Predict, giving a reason, how the value of the ΔS⦵reaction would be affected if (g) were used as a reactant.
Calculate the Gibbs free energy change, ΔG⦵, in kJ mol−1, for the reaction at 298 K. Use section 1 of the data booklet.
Calculate the equilibrium constant, Kc, for this reaction at 298 K. Use your answer to (d)(iii) and sections 1 and 2 of the data booklet.
(If you did not obtain an answer to (d)(iii) use a value of 2.0 kJ mol−1, although this is not the correct answer).
The following mechanism is proposed for a reaction:
A + B → C + D slow step
D + B → A + E fast step
Classify substances B and D as reactant, product, catalyst, or intermediate, based on the proposed mechanism.
Deduce the rate expression.
Calculate the initial rate of reaction for experiment 2, if measured under the same conditions.
This question is about the decomposition of hydrogen peroxide.
Hydrogen peroxide decomposes to water and oxygen when a catalyst such as potassium iodide, KI, is added.
2H2O2 (aq) O2 (g) + 2H2O (l)
Suggest why many chemicals, including hydrogen peroxide, are kept in brown bottles instead of clear colourless bottles.
In a laboratory experiment solutions of potassium iodide and hydrogen peroxide were mixed and the volume of oxygen generated was recorded. The volume was adjusted to 0 at t = 0.
The data for the first trial is given below.
Plot a graph on the axes below and from it determine the average rate of
formation of oxygen gas in cm3 O2 (g) s−1.
Average rate of reaction:
Two more trials (2 and 3) were carried out. The results are given below.
Determine the rate equation for the reaction and its overall order, using your answer from (b)(i).
Rate equation:
Overall order:
Additional experiments were carried out at an elevated temperature. On the axes below, sketch Maxwell–Boltzmann energy distribution curves at two temperatures T1 and T2, where T2 > T1.
Apart from a greater frequency of collisions, explain, by annotating your graphs in (b)(iii), why an increased temperature causes the rate of reaction to increase.
MnO2 is another possible catalyst for the reaction. State the IUPAC name for MnO2.
Comment on why peracetic acid, CH3COOOH, is always sold in solution with ethanoic acid and hydrogen peroxide.
H2O2 (aq) + CH3COOH (aq) ⇌ CH3COOOH (aq) + H2O (l)
Sodium percarbonate, 2Na2CO3•3H2O2, is an adduct of sodium carbonate and hydrogen peroxide and is used as a cleaning agent.
Mr (2Na2CO3•3H2O2) = 314.04
Calculate the percentage by mass of hydrogen peroxide in sodium percarbonate, giving your answer to two decimal places.
Propene is an important starting material for many products. The following shows some compounds which can be made from propene, C3H6.
Propene (C3H6) → C3H7Cl → C3H8O → C3H6O
Consider the conversion of propene to C3H7Cl.
An experiment was carried out to determine the order of reaction between one of the isomers of C3H7Cl and aqueous sodium hydroxide. The following results were obtained.
State the type of reaction.
State the IUPAC name of the major product.
Outline why it is the major product.
Write an equation for the reaction of the major product with aqueous sodium hydroxide to produce a C3H8O compound, showing structural formulas.
Determine the rate expression from the results, explaining your method.
Deduce the type of mechanism for the reaction of this isomer of C3H7Cl with aqueous sodium hydroxide.
Sketch the mechanism using curly arrows to represent the movement of electrons.
Write an equation for the complete combustion of the compound C3H8O formed in (a)(iv).
Determine the enthalpy of combustion of this compound, in kJ mol−1, using data from section 11 of the data booklet.
State the reagents for the conversion of the compound C3H8O formed in (a)(iv) into C3H6O.
Explain why the compound C3H8O, produced in (a)(iv), has a higher boiling point than compound C3H6O, produced in d(i).
Explain why the 1H NMR spectrum of C3H6O, produced in (d)(i), shows only one signal.
Propene is often polymerized. Draw a section of the resulting polymer, showing two repeating units.
Nitrogen dioxide and carbon monoxide react according to the following equation:
NO2(g) + CO(g) NO(g) + CO2(g) ΔH = –226 kJ
Experimental data shows the reaction is second order with respect to NO2 and zero order with respect to CO.
State the rate expression for the reaction.
The following mechanism is proposed for the reaction.
Identify the rate determining step giving your reason.
State one method that can be used to measure the rate for this reaction.
Sketch the relationship between the rate of reaction and the concentration of NO2.
The Arrhenius equation, , gives the relationship between the rate constant and temperature.
State how temperature affects activation energy.
Bromate and bromide ions react in acidic aqueous solution.
BrO3− (aq) + 5Br− (aq) + 6H+ (aq) → 3Br2 (l) + 3H2O (l)
The following rate data was collected.
Determine the rate expression for the reaction.
Determine the value and unit of the rate constant using the rate expression in (a).
Hybridization of hydrocarbons affects their reactivity.
Experiments were carried out to investigate the mechanism of reaction between 2-chloropentane and aqueous sodium hydroxide.
Distinguish between a sigma and pi bond.
Identify the hybridization of carbon in ethane, ethene and ethyne.
State, giving a reason, if but-1-ene exhibits cis-trans isomerism.
State the type of reaction which occurs between but-1-ene and hydrogen iodide at room temperature.
Explain the mechanism of the reaction between but-1-ene with hydrogen iodide, using curly arrows to represent the movement of electron pairs.
State, giving a reason, if the product of this reaction exhibits stereoisomerism.
Deduce the rate expression for this reaction.
Deduce the units of the rate constant.
Determine the initial rate of reaction in experiment 4.
Deduce, with a reason, the mechanism of the reaction between 2-chloropentane and sodium hydroxide.
Discuss the reason benzene is more reactive with an electrophile than a nucleophile.
A mixture of 1.00 mol SO2(g), 2.00 mol O2(g) and 1.00 mol SO3(g) is placed in a 1.00 dm3 container and allowed to reach equilibrium.
2SO2(g) + O2(g) 2SO3(g)
Nitrogen oxide is in equilibrium with dinitrogen dioxide.
2NO(g) N2O2(g) ΔHΘ < 0
Deduce, giving a reason, the effect of increasing the temperature on the concentration of N2O2.
A two-step mechanism is proposed for the formation of NO2(g) from NO(g) that involves an exothermic equilibrium process.
First step: 2NO(g) N2O2(g) fast
Second step: N2O2(g) + O2 (g) → 2NO2(g) slow
Deduce the rate expression for the mechanism.
The rate constant for a reaction doubles when the temperature is increased from 25.0 °C to 35 °C.
Calculate the activation energy, Ea, in kJ mol−1 for the reaction using section 1 and 2 of the data booklet.
Analytical chemistry uses instruments to separate, identify, and quantify matter.
Nitric oxide reacts with chlorine.
2NO (g) + Cl2 (g) → 2NOCl (g)
The following experimental data were obtained at 101.3 kPa and 263 K.
Menthol is an organic compound containing carbon, hydrogen and oxygen.
Outline how this spectrum is related to the energy levels in the hydrogen atom.
A sample of magnesium has the following isotopic composition.
Calculate the relative atomic mass of magnesium based on this data, giving your answer to two decimal places.
Complete combustion of 0.1595 g of menthol produces 0.4490 g of carbon dioxide and 0.1840 g of water. Determine the empirical formula of the compound showing your working.
0.150 g sample of menthol, when vaporized, had a volume of 0.0337 dm3 at 150 °C and 100.2 kPa. Calculate its molar mass showing your working.
Determine the molecular formula of menthol using your answers from parts (d)(i) and (ii).
Deduce the order of reaction with respect to Cl2 and NO.
State the rate expression for the reaction.
Calculate the value of the rate constant at 263 K.
When dinitrogen pentoxide, N2O5, is heated the colourless gas undergoes thermal decomposition to produce brown nitrogen dioxide:
N2O5 (g) → 2NO2 (g) + O2 (g)
Data for the decomposition at constant temperature is given.
Suggest how the extent of decomposition could be measured.
Plot the missing point on the graph and draw the best-fit line.
Outline why increasing the concentration of N2O5 increases the rate of reaction.
Write the rate expression for this reaction.
Calculate the value of the rate constant, k, giving its units.
Oxygen exists as two allotropes, diatomic oxygen, O2, and ozone, O3.
Draw a Lewis (electron dot) structure for ozone.
Discuss the relative length of the two O−O bonds in ozone.
Explain why there are frequencies of UV light that will dissociate O3 but not O2.
Explain, using equations, how the presence of results in a chain reaction that decreases the concentration of ozone in the stratosphere.