
HL Paper 2
Benzoic acid, C6H5COOH, is another derivative of benzene.
Identify the wavenumber of one peak in the IR spectrum of benzoic acid, using section 26 of the data booklet.
Identify the spectroscopic technique that is used to measure the bond lengths in solid benzoic acid.
Outline one piece of physical evidence for the structure of the benzene ring.
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
Outline why both C to O bonds in the conjugate base are the same length and suggest a value for them. Use section 10 of the data booklet.
The pH of an aqueous solution of benzoic acid at 298 K is 2.95. Determine the concentration of hydroxide ions in the solution, using section 2 of the data booklet.
Formulate the equation for the complete combustion of benzoic acid in oxygen using only integer coefficients.
The combustion reaction in (f)(ii) can also be classed as redox. Identify the atom that is oxidized and the atom that is reduced.
Suggest how benzoic acid, Mr = 122.13, forms an apparent dimer, Mr = 244.26, when dissolved in a non-polar solvent such as hexane.
State the reagent used to convert benzoic acid to phenylmethanol (benzyl alcohol), C6H5CH2OH.
A student performs a titration to determine the concentration of ethanoic acid, , in vinegar using potassium hydroxide.
The pH curve for the reaction is given.
Write a balanced equation for the reaction.
Identify the major species, other than water and potassium ions, at these points.
State a suitable indicator for this titration. Use section 22 of the data booklet
Suggest, giving a reason, which point on the curve is considered a buffer region.
State the expression for ethanoic acid.
Calculate the of the conjugate base of ethanoic acid using sections 2 and 21 of the data booklet.
In a titration, of vinegar required of potassium hydroxide to reach the end-point.
Calculate the concentration of ethanoic acid in the vinegar.
Potassium hydroxide solutions can react with carbon dioxide from the air. The solution was made one day prior to using it in the titration.
State the type of error that would result from the student’s approach.
Potassium hydroxide solutions can react with carbon dioxide from the air. The solution was made one day prior to using it in the titration.
Predict, giving a reason, the effect of this error on the calculated concentration of ethanoic acid in 5(e).
Magnetite, Fe3O4, is another ore of iron that contains both Fe2+ and Fe3+.
Iron exists as several isotopes.
Deduce the ratio of Fe2+:Fe3+ in Fe3O4.
State the type of spectroscopy that could be used to determine their relative abundances.
State the number of protons, neutrons and electrons in each species.
Iron has a relatively small specific heat capacity; the temperature of a 50 g sample rises by 44.4°C when it absorbs 1 kJ of heat energy.
Determine the specific heat capacity of iron, in J g−1 K−1. Use section 1 of the data booklet.
A voltaic cell is set up between the Fe2+ (aq) | Fe (s) and Fe3+ (aq) | Fe2+ (aq) half-cells.
Deduce the equation and the cell potential of the spontaneous reaction. Use section 24 of the data booklet.
The figure shows an apparatus that could be used to electroplate iron with zinc. Label the figure with the required substances.
Outline why, unlike typical transition metals, zinc compounds are not coloured.
Transition metals like iron can form complex ions. Discuss the bonding between transition metals and their ligands in terms of acid-base theory.
The concentration of a solution of a weak acid, such as ethanedioic acid, can be determined
by titration with a standard solution of sodium hydroxide, NaOH (aq).
5.00 g of an impure sample of hydrated ethanedioic acid, (COOH)2•2H2O, was dissolved in water to make 1.00 dm3 of solution. 25.0 cm3 samples of this solution were titrated against a 0.100 mol dm-3 solution of sodium hydroxide using a suitable indicator.
(COOH)2 (aq) + 2NaOH (aq) → (COONa)2 (aq) + 2H2O (l)
The mean value of the titre was 14.0 cm3.
(i) Suggest a suitable indicator for this titration. Use section 22 of the data booklet.
(ii) Calculate the amount, in mol, of NaOH in 14.0 cm3 of 0.100 mol dm-3 solution.
(iii) Calculate the amount, in mol, of ethanedioic acid in each 25.0 cm3 sample.
(iv) Determine the percentage purity of the hydrated ethanedioic acid sample.
Draw the Lewis (electron dot) structure of the ethanedioate ion, –OOCCOO–.
Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest a value for them. Use section 10 of the data booklet.
Explain how ethanedioate ions act as ligands.
Analytical chemistry uses instruments to separate, identify, and quantify matter.
Nitric oxide reacts with chlorine.
2NO (g) + Cl2 (g) → 2NOCl (g)
The following experimental data were obtained at 101.3 kPa and 263 K.
Menthol is an organic compound containing carbon, hydrogen and oxygen.
Outline how this spectrum is related to the energy levels in the hydrogen atom.
A sample of magnesium has the following isotopic composition.
Calculate the relative atomic mass of magnesium based on this data, giving your answer to two decimal places.
Complete combustion of 0.1595 g of menthol produces 0.4490 g of carbon dioxide and 0.1840 g of water. Determine the empirical formula of the compound showing your working.
0.150 g sample of menthol, when vaporized, had a volume of 0.0337 dm3 at 150 °C and 100.2 kPa. Calculate its molar mass showing your working.
Determine the molecular formula of menthol using your answers from parts (d)(i) and (ii).
Deduce the order of reaction with respect to Cl2 and NO.
State the rate expression for the reaction.
Calculate the value of the rate constant at 263 K.
Urea, (H2N)2CO, is excreted by mammals and can be used as a fertilizer.
Urea can also be made by the direct combination of ammonia and carbon dioxide gases.
2NH3(g) + CO2(g) (H2N)2CO(g) + H2O(g) ΔH < 0
Calculate the percentage by mass of nitrogen in urea to two decimal places using section 6 of the data booklet.
Suggest how the percentage of nitrogen affects the cost of transport of fertilizers giving a reason.
The structural formula of urea is shown.
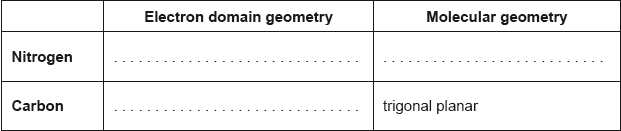
Predict the electron domain and molecular geometries at the nitrogen and carbon atoms, applying the VSEPR theory.
Urea can be made by reacting potassium cyanate, KNCO, with ammonium chloride, NH4Cl.
KNCO(aq) + NH4Cl(aq) → (H2N)2CO(aq) + KCl(aq)
Determine the maximum mass of urea that could be formed from 50.0 cm3 of 0.100 mol dm−3 potassium cyanate solution.
State the equilibrium constant expression, Kc.
Predict, with a reason, the effect on the equilibrium constant, Kc, when the temperature is increased.
Determine an approximate order of magnitude for Kc, using sections 1 and 2 of the data booklet. Assume ΔGΘ for the forward reaction is approximately +50 kJ at 298 K.
Suggest one reason why urea is a solid and ammonia a gas at room temperature.
Sketch two different hydrogen bonding interactions between ammonia and water.
The combustion of urea produces water, carbon dioxide and nitrogen.
Formulate a balanced equation for the reaction.
Calculate the maximum volume of CO2, in cm3, produced at STP by the combustion of 0.600 g of urea, using sections 2 and 6 of the data booklet.
Describe the bond formation when urea acts as a ligand in a transition metal complex ion.
The C–N bonds in urea are shorter than might be expected for a single C–N bond. Suggest, in terms of electrons, how this could occur.
The mass spectrum of urea is shown below.
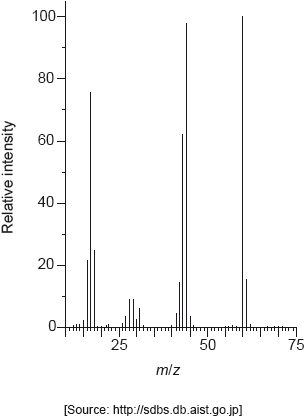
Identify the species responsible for the peaks at m/z = 60 and 44.
The IR spectrum of urea is shown below.
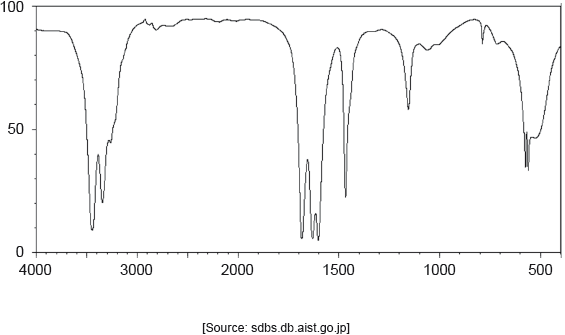
Identify the bonds causing the absorptions at 3450 cm−1 and 1700 cm−1 using section 26 of the data booklet.
Predict the number of signals in the 1H NMR spectrum of urea.
Predict the splitting pattern of the 1H NMR spectrum of urea.
Outline why TMS (tetramethylsilane) may be added to the sample to carry out 1H NMR spectroscopy and why it is particularly suited to this role.
This question is about sodium and its compounds.
The Born-Haber cycle for sodium oxide is shown (not to scale).
Sodium peroxide is used in diving apparatus to produce oxygen from carbon dioxide.
2Na2O2 (s) + 2CO2 (g) → 2Na2CO3 (s) + O2 (g)
Plot the relative values of the first four ionization energies of sodium.
Outline why the alkali metals (group 1) have similar chemical properties.
Describe the structure and bonding in solid sodium oxide.
Calculate values for the following changes using section 8 of the data booklet.
ΔHatomisation (Na) = 107 kJ mol−1
ΔHatomisation (O) = 249 kJ mol−1
O2(g) → O2- (g):
Na (s) → Na+ (g):
The standard enthalpy of formation of sodium oxide is −414 kJ mol−1. Determine the lattice enthalpy of sodium oxide, in kJ mol−1, using section 8 of the data booklet and your answers to (d)(i).
(If you did not get answers to (d)(i), use +850 kJ mol−1 and +600 kJ mol−1 respectively, but these are not the correct answers.)
Justify why K2O has a lower lattice enthalpy (absolute value) than Na2O.
Write equations for the separate reactions of solid sodium oxide and solid phosphorus(V) oxide with excess water and differentiate between the solutions formed.
Sodium oxide, Na2O:
Phosphorus(V) oxide, P4O10:
Differentiation:
Sodium peroxide, Na2O2, is formed by the reaction of sodium oxide with oxygen.
2Na2O (s) + O2 (g) → 2Na2O2 (s)
Calculate the percentage yield of sodium peroxide if 5.00g of sodium oxide produces 5.50g of sodium peroxide.
Determine the enthalpy change, ΔH, in kJ, for this reaction using data from the table and section 12 of the data booklet.
Outline why bond enthalpy values are not valid in calculations such as that in (g)(i).
An allotrope of molecular oxygen is ozone. Compare, giving a reason, the bond enthalpies of the O to O bonds in O2 and O3.
Outline why a real gas differs from ideal behaviour at low temperature and high pressure.
The reaction of sodium peroxide with excess water produces hydrogen peroxide and one other sodium compound. Suggest the formula of this compound.
State the oxidation number of carbon in sodium carbonate, Na2CO3.
Copper forms two chlorides, copper(I) chloride and copper(II) chloride.
Two electrolysis cells were assembled using graphite electrodes and connected in series as shown.
Copper(I) chloride undergoes a disproportionation reaction, producing copper(II) chloride and copper.
2Cu+ (aq) → Cu (s) + Cu2+ (aq)
Dilute copper(II) chloride solution is light blue, while copper(I) chloride solution is colourless.
State the electron configuration of the Cu+ ion.
Copper(II) chloride is used as a catalyst in the production of chlorine from hydrogen chloride.
4HCl (g) + O2 (g) → 2Cl2 (g) + 2H2O (g)
Calculate the standard enthalpy change, ΔHθ, in kJ, for this reaction, using section 12 of the data booklet.
The diagram shows the Maxwell–Boltzmann distribution and potential energy profile for the reaction without a catalyst.
Annotate both charts to show the activation energy for the catalysed reaction, using the label Ea (cat).
Explain how the catalyst increases the rate of the reaction.
Solid copper(II) chloride absorbs moisture from the atmosphere to form a hydrate of formula CuCl2•H2O.
A student heated a sample of hydrated copper(II) chloride, in order to determine the value of . The following results were obtained:
Mass of crucible = 16.221 g
Initial mass of crucible and hydrated copper(II) chloride = 18.360 g
Final mass of crucible and anhydrous copper(II) chloride = 17.917 g
Determine the value of .
State how current is conducted through the wires and through the electrolyte.
Wires:
Electrolyte:
Write the half-equation for the formation of gas bubbles at electrode 1.
Bubbles of gas were also observed at another electrode. Identify the electrode and the gas.
Electrode number (on diagram):
Name of gas:
Deduce the half-equation for the formation of the gas identified in (c)(iii).
Determine the enthalpy of solution of copper(II) chloride, using data from sections 18 and 20 of the data booklet.
The enthalpy of hydration of the copper(II) ion is −2161 kJ mol−1.
Calculate the cell potential at 298 K for the disproportionation reaction, in V, using section 24 of the data booklet.
Comment on the spontaneity of the disproportionation reaction at 298 K.
Calculate the standard Gibbs free energy change, ΔGθ, to two significant figures, for the disproportionation at 298 K. Use your answer from (e)(i) and sections 1 and 2 of the data booklet.
Suggest, giving a reason, whether the entropy of the system increases or decreases during the disproportionation.
Deduce, giving a reason, the sign of the standard enthalpy change, ΔHθ, for the disproportionation reaction at 298 K.
Predict, giving a reason, the effect of increasing temperature on the stability of copper(I) chloride solution.
Describe how the blue colour is produced in the Cu(II) solution. Refer to section 17 of the data booklet.
Deduce why the Cu(I) solution is colourless.
When excess ammonia is added to copper(II) chloride solution, the dark blue complex ion, [Cu(NH3)4(H2O)2]2+, forms.
State the molecular geometry of this complex ion, and the bond angles within it.
Molecular geometry:
Bond angles:
Examine the relationship between the Brønsted–Lowry and Lewis definitions of a base, referring to the ligands in the complex ion [CuCl4]2−.
A student determined the percentage of the active ingredient magnesium hydroxide, Mg(OH)2, in a 1.24 g antacid tablet.
The antacid tablet was added to 50.00 cm3 of 0.100 mol dm−3 sulfuric acid, which was in excess.
Outline why repeating quantitative measurements is important.
Biochemical oxygen demand (BOD) can be determined by the Winkler Method.
A 25.00 cm3 sample of water was treated according to the Winkler Method.
Step I: 2Mn2+ (aq) + O2 (g) + 4OH− (aq) → 2MnO2 (s) + 2H2O (l)
Step II: MnO2 (s) + 2I− (aq) + 4H+ (aq) → Mn2+ (aq) + I2 (aq) + 2H2O (l)
Step III: 2S2O32− (aq) + I2 (aq) → 2I− (aq) + S4O62− (aq)
The iodine produced was titrated with 37.50 cm3 of 5.000 × 10−4 mol dm−3 Na2S2O3.
Outline what is measured by BOD.
A student dissolved 0.1240 ± 0.0001 g of Na2S2O3 to make 1000.0 ± 0.4 cm3 of solution to use in the Winkler Method.
Determine the percentage uncertainty in the molar concentration.
Calculate the amount, in moles of Na2S2O3 used in the titration.
Deduce the mole ratio of O2 consumed in step I to S2O32− used in step III.
Calculate the concentration of dissolved oxygen, in mol dm−3, in the sample.
The three steps of the Winkler Method are redox reactions.
Deduce the reduction half-equation for step II.
Suggest a reason that the Winkler Method used to measure biochemical oxygen demand (BOD) must be done at constant temperature.
Vanadium has a number of different oxidation states.
Electrode potentials for the reactions of vanadium and other species are shown below.
Determine the oxidation state of vanadium in each of the following species.
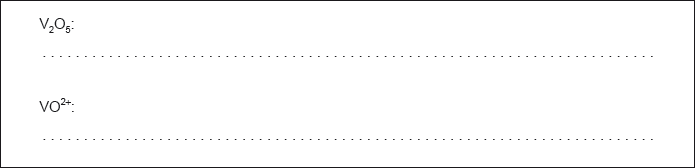
Identify, from the table, a non-vanadium species that can reduce VO2+(aq) to V3+(aq) but no further.
Identify, from the table, a non-vanadium species that could convert to V2+(aq).
Formulate an equation for the reaction between VO2+(aq) and V2+(aq) in acidic solution to form V3+(aq).
Comment on the spontaneity of this reaction by calculating a value for using the data given in (b) and in section 1 of the data booklet.
White phosphorus is an allotrope of phosphorus and exists as P4.
An equilibrium exists between PCl3 and PCl5.
PCl3 (g) + Cl2 (g) PCl5 (g)
Sketch the Lewis (electron dot) structure of the P4 molecule, containing only single bonds.
Write an equation for the reaction of white phosphorus (P4) with chlorine gas to form phosphorus trichloride (PCl3).
Deduce the electron domain and molecular geometry using VSEPR theory, and estimate the Cl–P–Cl bond angle in PCl3.
Outline the reason why PCl5 is a non-polar molecule, while PCl4F is polar.
Calculate the standard enthalpy change (ΔH⦵) for the forward reaction in kJ mol−1.
ΔH⦵f PCl3 (g) = −306.4 kJ mol−1
ΔH⦵f PCl5 (g) = −398.9 kJ mol−1
Calculate the entropy change, ΔS, in J K−1 mol−1, for this reaction.
Chemistry 2e, Chpt. 21 Nuclear Chemistry, Appendix G: Standard Thermodynamic Properties for Selected Substances https://openstax.org/books/chemistry-2e/pages/g-standard-thermodynamic-properties-for- selectedsubstances# page_667adccf-f900-4d86-a13d-409c014086ea © 1999-2021, Rice University. Except where otherwise noted, textbooks on this site are licensed under a Creative Commons Attribution 4.0 International License. (CC BY 4.0) https://creativecommons.org/licenses/by/4.0/.
Calculate the Gibbs free energy change (ΔG), in kJ mol−1, for this reaction at 25 °C. Use section 1 of the data booklet.
If you did not obtain an answer in c(i) or c(ii) use −87.6 kJ mol−1 and −150.5 J mol−1 K−1 respectively, but these are not the correct answers.
Determine the equilibrium constant, K, for this reaction at 25 °C, referring to section 1 of the data booklet.
If you did not obtain an answer in (c)(iii), use ΔG = –43.5 kJ mol−1, but this is not the correct answer.
State the equilibrium constant expression, Kc, for this reaction.
State, with a reason, the effect of an increase in temperature on the position of this equilibrium.
Phosphoric acid, H3PO4 can form three different salts depending on the extent of neutralisation by sodium hydroxide.
Formulate an equation for the reaction of one mole of phosphoric acid with one mole of sodium hydroxide.
Formulate two equations to show the amphiprotic nature of H2PO4−.
Calculate the concentration of H3PO4 if 25.00 cm3 is completely neutralised by the addition of 28.40 cm3 of 0.5000 mol dm−3 NaOH.
Outline the reasons that sodium hydroxide is considered a Brønsted–Lowry and Lewis base.
A 4.406 g sample of a compound containing only C, H and O was burnt in excess oxygen. 8.802 g of CO2 and 3.604 g of H2O were produced.
The following spectrums show the Infrared spectra of propan-1-ol, propanal and propanoic acid.
NIST Mass Spectrometry Data Center Collection © 2021 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved. Available at: https://webbook.nist.gov/cgi/cbook.cgi?ID=C71238&Units=SI&Type=IRSPEC&Index=3#IR-SPEC [Accessed 6 May 2020]. Source adapted.
NIST Mass Spectrometry Data Center Collection © 2021 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. Available at: https://webbook.nist.gov/cgi/cbook.cgi?ID=C79094&Units=SI&Mask=80#IR-Spec [Accessed 6 May 2020]. Source adapted.
NIST Mass Spectrometry Data Center Collection © 2021 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. Available at: https://webbook.nist.gov/cgi/cbook.cgi?Name=propanal&Units=SI&cIR=on&cTZ=on#IRSpec [Accessed 6 May 2020]. Source adapted.
Determine the empirical formula of the compound using section 6 of the data booklet.
Determine the molecular formula of this compound if its molar mass is 88.12 g mol−1. If you did not obtain an answer in (a) use CS, but this is not the correct answer.
Identify each compound from the spectra given, use absorptions from the range of 1700 cm−1 to 3500 cm−1. Explain the reason for your choice, referring to section 26 of the data booklet.
Predict the number of 1H NMR signals, and splitting pattern of the –CH3 seen for propanone (CH3COCH3) and propanal (CH3CH2CHO).
Predict the fragment that is responsible for a m/z of 31 in the mass spectrum of propan‑1‑ol. Use section 28 of the data booklet.
This reaction is used in the manufacture of sulfuric acid.
2SO2 (g) + O2 (g) 2SO3 (g) Kc = 280 at 1000 K
State why this equilibrium reaction is considered homogeneous.
Predict, giving your reason, the sign of the standard entropy change of the forward reaction.
Calculate the standard Gibbs free energy change, ΔGΘ, in kJ, for this reaction at 1000 K. Use sections 1 and 2 of the data booklet.
Predict, giving your reasons, whether the forward reaction is endothermic or exothermic. Use your answers to (b) and (c).
0.200 mol sulfur dioxide, 0.300 mol oxygen and 0.500 mol sulfur trioxide were mixed in a 1.00 dm3 flask at 1000 K.
Predict the direction of the reaction showing your working.
When heated in air, magnesium ribbon reacts with oxygen to form magnesium oxide.
The reaction in (a)(i) was carried out in a crucible with a lid and the following data was recorded:
Mass of crucible and lid = 47.372 ±0.001 g
Mass of crucible, lid and magnesium ribbon before heating = 53.726 ±0.001 g
Mass of crucible, lid and product after heating = 56.941 ±0.001 g
When magnesium is burnt in air, some of it reacts with nitrogen to form magnesium nitride according to the equation:
3 Mg (s) + N2 (g) → Mg3N2 (s)
The presence of magnesium nitride can be demonstrated by adding water to the product. It is hydrolysed to form magnesium hydroxide and ammonia.
Most nitride ions are 14N3–.
Write a balanced equation for the reaction that occurs.
Identify a metal, in the same period as magnesium, that does not form a basic oxide.
Calculate the amount of magnesium, in mol, that was used.
Determine the percentage uncertainty of the mass of product after heating.
Assume the reaction in (a)(i) is the only one occurring and it goes to completion, but some product has been lost from the crucible. Deduce the percentage yield of magnesium oxide in the crucible.
Evaluate whether this, rather than the loss of product, could explain the yield found in (b)(iii).
Suggest an explanation, other than product being lost from the crucible or reacting with nitrogen, that could explain the yield found in (b)(iii).
Calculate coefficients that balance the equation for the following reaction.
Ammonia is added to water that contains a few drops of an indicator. Identify an indicator that would change colour. Use sections 21 and 22 of the data booklet.
Determine the oxidation state of nitrogen in Mg3N2 and in NH3.
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
State the number of subatomic particles in this ion.
Some nitride ions are 15N3–. State the term that describes the relationship between 14N3– and 15N3–.
The nitride ion and the magnesium ion are isoelectronic (they have the same electron configuration). Determine, giving a reason, which has the greater ionic radius.
Suggest, giving a reason, whether magnesium or nitrogen would have the greater sixth ionization energy.
Suggest two reasons why atoms are no longer regarded as the indivisible units of matter.
State the types of bonding in magnesium, oxygen and magnesium oxide, and how the valence electrons produce these types of bonding.
Chlorine undergoes many reactions.
of manganese(IV) oxide was added to of .
Chlorine gas reacts with water to produce hypochlorous acid and hydrochloric acid.
is a common chlorofluorocarbon, .
State the full electron configuration of the chlorine atom.
State, giving a reason, whether the chlorine atom or the chloride ion has a larger radius.
Outline why the chlorine atom has a smaller atomic radius than the sulfur atom.
The mass spectrum of chlorine is shown.
NIST Mass Spectrometry Data Center Collection © 2014 copyright by the U.S. Secretary of Commerce on behalf of the United States of America. All rights reserved.
Outline the reason for the two peaks at and .
Explain the presence and relative abundance of the peak at .
Calculate the amount, in , of manganese(IV) oxide added.
Determine the limiting reactant, showing your calculations.
Determine the excess amount, in , of the other reactant.
Calculate the volume of chlorine, in , produced if the reaction is conducted at standard temperature and pressure (STP). Use section 2 of the data booklet.
State the oxidation state of manganese in and .
Deduce, referring to oxidation states, whether is an oxidizing or reducing agent.
Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid.
State the formula of the conjugate base of hypochlorous acid.
Calculate the concentration of in a solution with a .
State the type of reaction occurring when ethane reacts with chlorine to produce chloroethane.
Predict, giving a reason, whether ethane or chloroethane is more reactive.
Explain the mechanism of the reaction between chloroethane and aqueous sodium hydroxide, , using curly arrows to represent the movement of electron pairs.
Ethoxyethane (diethyl ether) can be used as a solvent for this conversion.
Draw the structural formula of ethoxyethane
Deduce the number of signals and chemical shifts with splitting patterns in the 1H NMR spectrum of ethoxyethane. Use section 27 of the data booklet.
Calculate the percentage by mass of chlorine in .
Comment on how international cooperation has contributed to the lowering of emissions responsible for ozone depletion.
s produce chlorine radicals. Write two successive propagation steps to show how chlorine radicals catalyse the depletion of ozone.
Magnesium is a group 2 metal which exists as a number of isotopes and forms many compounds.
Magnesium ions produce no emission or absorption lines in the visible region of the electromagnetic spectrum. Suggest why most magnesium compounds tested in a school laboratory show traces of yellow in the flame.
(i) Explain the convergence of lines in a hydrogen emission spectrum.
(ii) State what can be determined from the frequency of the convergence limit.
Magnesium chloride can be electrolysed.
(i) Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922K and 987K respectively.
(ii) Identify the type of reaction occurring at the cathode (negative electrode).
(iii) State the products when a very dilute aqueous solution of magnesium chloride is electrolysed.
Standard electrode potentials are measured relative to the standard hydrogen electrode. Describe a standard hydrogen electrode.
A magnesium half-cell, Mg(s)/Mg2+(aq), can be connected to a copper half-cell, Cu(s)/Cu2+(aq).
(i) Formulate an equation for the spontaneous reaction that occurs when the circuit is completed.
(ii) Determine the standard cell potential, in V, for the cell. Refer to section 24 of the data booklet.
(iii) Predict, giving a reason, the change in cell potential when the concentration of copper ions increases.
This question is about the decomposition of hydrogen peroxide.
Hydrogen peroxide decomposes to water and oxygen when a catalyst such as potassium iodide, KI, is added.
2H2O2 (aq) O2 (g) + 2H2O (l)
Suggest why many chemicals, including hydrogen peroxide, are kept in brown bottles instead of clear colourless bottles.
In a laboratory experiment solutions of potassium iodide and hydrogen peroxide were mixed and the volume of oxygen generated was recorded. The volume was adjusted to 0 at t = 0.
The data for the first trial is given below.
Plot a graph on the axes below and from it determine the average rate of
formation of oxygen gas in cm3 O2 (g) s−1.
Average rate of reaction:
Two more trials (2 and 3) were carried out. The results are given below.
Determine the rate equation for the reaction and its overall order, using your answer from (b)(i).
Rate equation:
Overall order:
Additional experiments were carried out at an elevated temperature. On the axes below, sketch Maxwell–Boltzmann energy distribution curves at two temperatures T1 and T2, where T2 > T1.
Apart from a greater frequency of collisions, explain, by annotating your graphs in (b)(iii), why an increased temperature causes the rate of reaction to increase.
MnO2 is another possible catalyst for the reaction. State the IUPAC name for MnO2.
Comment on why peracetic acid, CH3COOOH, is always sold in solution with ethanoic acid and hydrogen peroxide.
H2O2 (aq) + CH3COOH (aq) ⇌ CH3COOOH (aq) + H2O (l)
Sodium percarbonate, 2Na2CO3•3H2O2, is an adduct of sodium carbonate and hydrogen peroxide and is used as a cleaning agent.
Mr (2Na2CO3•3H2O2) = 314.04
Calculate the percentage by mass of hydrogen peroxide in sodium percarbonate, giving your answer to two decimal places.
Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Now consider the second stage of the reaction.
CO (g) + 2H2 (g) CH3OH (l) ΔH⦵ = –129 kJ
Hydrogen peroxide can react with methane and oxygen to form methanol. This reaction can occur below 50°C if a gold nanoparticle catalyst is used.
Methanol is usually manufactured from methane in a two-stage process.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
CO (g) + 2H2 (g) CH3OH (l)
Consider the first stage of the reaction.
CH4 (g) + H2O (g) CO (g) + 3H2 (g)
The diagram shows the Maxwell-Boltzmann curve for the uncatalyzed reaction.
Draw a distribution curve at a lower temperature (T2) and show on the diagram how the addition of a catalyst enables the reaction to take place more rapidly than at T1.
The hydrogen peroxide could cause further oxidation of the methanol. Suggest a possible oxidation product.
Determine the overall equation for the production of methanol.
8.00 g of methane is completely converted to methanol. Calculate, to three significant figures, the final volume of hydrogen at STP, in dm3. Use sections 2 and 6 of the data booklet.
Determine the enthalpy change, ΔH, in kJ. Use section 11 of the data booklet.
Bond enthalpy of CO = 1077 kJ mol−1.
State one reason why you would expect the value of ΔH calculated from the values, given in section 12 of data booklet, to differ from your answer to (d)(i).
State the expression for Kc for this stage of the reaction.
State and explain the effect of increasing temperature on the value of Kc.
The equilibrium constant, Kc, has a value of 1.01 at 298 K.
Calculate ΔG⦵, in kJ mol–1, for this reaction. Use sections 1 and 2 of the data booklet.
Calculate a value for the entropy change, ΔS⦵, in J K–1 mol–1 at 298 K. Use your answers to (e)(i) and section 1 of the data booklet.
If you did not get answers to (e)(i) use –1 kJ, but this is not the correct answer.
Justify the sign of ΔS with reference to the equation.
Predict, giving a reason, how a change in temperature from 298 K to 273 K would affect the spontaneity of the reaction.
Limestone can be converted into a variety of useful commercial products through the lime cycle. Limestone contains high percentages of calcium carbonate, CaCO3.
Thermodynamic data for the decomposition of calcium carbonate is given.
The second step of the lime cycle produces calcium hydroxide, Ca(OH)2.
Calcium hydroxide reacts with carbon dioxide to reform calcium carbonate.
Ca(OH)2 (aq) + CO2 (g) → CaCO3 (s) + H2O (l)
Calcium carbonate is heated to produce calcium oxide, CaO.
CaCO3 (s) → CaO (s) + CO2 (g)
Calculate the volume of carbon dioxide produced at STP when 555 g of calcium carbonate decomposes. Use sections 2 and 6 of the data booklet.
Calculate the enthalpy change of reaction, ΔH, in kJ, for the decomposition of calcium carbonate.
Calculate the change in entropy, ΔS, in J K−1, for the decomposition of calcium carbonate.
Determine the temperature, in K, at which the decomposition of calcium carbonate becomes spontaneous, using b(i), b(ii) and section 1 of the data booklet.
(If you do not have answers for b(i) and b(ii), use ΔH = 190 kJ and ΔS = 180 J K−1, but these are not the correct answers.)
Sketch an energy profile for the decomposition of calcium carbonate based on your answer to b(i), labelling the axes and activation energy, Ea.
State how adding a catalyst to the reaction would impact the enthalpy change of reaction, ΔH, and the activation energy, Ea.
Write the equation for the reaction of Ca(OH)2 (aq) with hydrochloric acid, HCl (aq).
Determine the volume, in dm3, of 0.015 mol dm−3 calcium hydroxide solution needed to neutralize 35.0 cm3 of 0.025 mol dm−3 HCl (aq).
Saturated calcium hydroxide solution is used to test for carbon dioxide. Calculate the pH of a 2.33 × 10−2 mol dm−3 solution of calcium hydroxide, a strong base.
Determine the mass, in g, of CaCO3 (s) produced by reacting 2.41 dm3 of 2.33 × 10−2 mol dm−3 of Ca(OH)2 (aq) with 0.750 dm3 of CO2 (g) at STP.
2.85 g of CaCO3 was collected in the experiment in d(i). Calculate the percentage yield of CaCO3.
(If you did not obtain an answer to d(i), use 4.00 g, but this is not the correct value.)
Outline how one calcium compound in the lime cycle can reduce a problem caused by acid deposition.
Propene is an important starting material for many products. The following shows some compounds which can be made from propene, C3H6.
Propene (C3H6) → C3H7Cl → C3H8O → C3H6O
Consider the conversion of propene to C3H7Cl.
An experiment was carried out to determine the order of reaction between one of the isomers of C3H7Cl and aqueous sodium hydroxide. The following results were obtained.
State the type of reaction.
State the IUPAC name of the major product.
Outline why it is the major product.
Write an equation for the reaction of the major product with aqueous sodium hydroxide to produce a C3H8O compound, showing structural formulas.
Determine the rate expression from the results, explaining your method.
Deduce the type of mechanism for the reaction of this isomer of C3H7Cl with aqueous sodium hydroxide.
Sketch the mechanism using curly arrows to represent the movement of electrons.
Write an equation for the complete combustion of the compound C3H8O formed in (a)(iv).
Determine the enthalpy of combustion of this compound, in kJ mol−1, using data from section 11 of the data booklet.
State the reagents for the conversion of the compound C3H8O formed in (a)(iv) into C3H6O.
Explain why the compound C3H8O, produced in (a)(iv), has a higher boiling point than compound C3H6O, produced in d(i).
Explain why the 1H NMR spectrum of C3H6O, produced in (d)(i), shows only one signal.
Propene is often polymerized. Draw a section of the resulting polymer, showing two repeating units.
Rhenium, Re, was the last element with a stable isotope to be isolated.
Before its isolation, scientists predicted the existence of rhenium and some of its properties.
One chloride of rhenium has the empirical formula ReCl3.
Rhenium forms salts containing the perrhenate(VII) ion, ReO4−.
The stable isotope of rhenium contains 110 neutrons.
State the nuclear symbol notation for this isotope.
Suggest the basis of these predictions.
A scientist wants to investigate the catalytic properties of a thin layer of rhenium metal on a graphite surface.
Describe an electrochemical process to produce a layer of rhenium on graphite.
Predict two other chemical properties you would expect rhenium to have, given its position in the periodic table.
Describe how the relative reactivity of rhenium, compared to silver, zinc, and copper, can be established using pieces of rhenium and solutions of these metal sulfates.
State the name of this compound, applying IUPAC rules.
Calculate the percentage, by mass, of rhenium in ReCl3.
Suggest why the existence of salts containing an ion with this formula could be predicted. Refer to section 6 of the data booklet.
Deduce the coefficients required to complete the half-equation.
ReO4− (aq) + ____H+ (aq) + ____e− ⇌ [Re(OH)2]2+ (aq) + ____H2O (l) Eθ = +0.36 V
Predict, giving a reason, whether the reduction of ReO4− to [Re(OH)2]2+ would oxidize Fe2+ to Fe3+ in aqueous solution. Use section 24 of the data booklet.
3.26 g of iron powder are added to 80.0 cm3 of 0.200 mol dm−3 copper(II) sulfate solution. The following reaction occurs:
Fe (s) + CuSO4 (aq) → FeSO4 (aq) + Cu (s)
Determine the limiting reactant showing your working.
The mass of copper obtained experimentally was 0.872 g. Calculate the percentage yield of copper.
The reaction was carried out in a calorimeter. The maximum temperature rise of the solution was 7.5 °C.
Calculate the enthalpy change, ΔH, of the reaction, in kJ, assuming that all the heat released was absorbed by the solution. Use sections 1 and 2 of the data booklet.
State another assumption you made in (b)(i).
The only significant uncertainty is in the temperature measurement.
Determine the absolute uncertainty in the calculated value of ΔH if the uncertainty in the temperature rise was ±0.2 °C.
Sketch a graph of the concentration of iron(II) sulfate, FeSO4, against time as the reaction proceeds.
Outline how the initial rate of reaction can be determined from the graph in part (c)(i).
Explain, using the collision theory, why replacing the iron powder with a piece of iron of the same mass slows down the rate of the reaction.
A student electrolyzed aqueous iron(II) sulfate, FeSO4 (aq), using platinum electrodes. State half-equations for the reactions at the electrodes, using section 24 of the data booklet.
The thermal decomposition of dinitrogen monoxide occurs according to the equation:
2N2O (g) → 2N2 (g) + O2 (g)
The reaction can be followed by measuring the change in total pressure, at constant temperature, with time.
The x-axis and y-axis are shown with arbitrary units.
This decomposition obeys the rate expression:
= k[N2O]
Explain why, as the reaction proceeds, the pressure increases by the amount shown.
Outline, in terms of collision theory, how a decrease in pressure would affect the rate of reaction.
Deduce how the rate of reaction at t = 2 would compare to the initial rate.
It has been suggested that the reaction occurs as a two-step process:
Step 1: N2O (g) → N2 (g) + O (g)
Step 2: N2O (g) + O (g) → N2 (g) + O2 (g)
Explain how this could support the observed rate expression.
The experiment is repeated using the same amount of dinitrogen monoxide in the same apparatus, but at a lower temperature.
Sketch, on the axes in question 2, the graph that you would expect.
The experiment gave an error in the rate because the pressure gauge was inaccurate.
Outline whether repeating the experiment, using the same apparatus, and averaging the results would reduce the error.
The graph below shows the Maxwell–Boltzmann distribution of molecular energies at a particular temperature.
The rate at which dinitrogen monoxide decomposes is significantly increased by a metal oxide catalyst.
Annotate and use the graph to outline why a catalyst has this effect.
Determine the standard entropy change, in J K−1, for the decomposition of dinitrogen monoxide.
2N2O (g) → 2N2 (g) + O2 (g)
Dinitrogen monoxide has a positive standard enthalpy of formation, ΔHfθ.
Deduce, giving reasons, whether altering the temperature would change the spontaneity of the decomposition reaction.
An organic compound containing carbon, hydrogen and oxygen has 62.02 % carbon and 10.43 % hydrogen by mass.
Determine the empirical formula of the compound, showing your working.
The infrared spectrum of the compound is shown. Deduce the functional group of the compound.
The mass spectrum of the compound is shown. Deduce the relative molecular mass of the compound.
The compound could not be oxidized using acidifi ed potassium dichromate(VI).
Deduce the structural formula of the compound.
Carbonated water is produced when carbon dioxide is dissolved in water under pressure. The following equilibria are established.
Equilibrium (1) CO2 (g) CO2 (aq)
Equilibrium (2) CO2 (aq) + H2O (l) H+ (aq) + HCO3− (aq)
Carbon dioxide acts as a weak acid.
Soda water has sodium hydrogencarbonate, NaHCO3, dissolved in the carbonated water.
Distinguish between a weak and strong acid.
Weak acid:
Strong acid:
The hydrogencarbonate ion, produced in Equilibrium (2), can also act as an acid.
State the formula of its conjugate base.
When a bottle of carbonated water is opened, these equilibria are disturbed.
State, giving a reason, how a decrease in pressure affects the position of Equilibrium (1).
At 298 K the concentration of aqueous carbon dioxide in carbonated water is 0.200 mol dm−3 and the pKa for Equilibrium (2) is 6.36.
Calculate the pH of carbonated water.
Identify the type of bonding in sodium hydrogencarbonate.
Between sodium and hydrogencarbonate:
Between hydrogen and oxygen in hydrogencarbonate:
Predict, referring to Equilibrium (2), how the added sodium hydrogencarbonate affects the pH.(Assume pressure and temperature remain constant.)
100.0cm3 of soda water contains 3.0 × 10−2g NaHCO3.
Calculate the concentration of NaHCO3 in mol dm−3.
The uncertainty of the 100.0cm3 volumetric flask used to make the solution was ±0.6cm3.
Calculate the maximum percentage uncertainty in the mass of NaHCO3 so that the concentration of the solution is correct to ±1.0 %.
The reaction of the hydroxide ion with carbon dioxide and with the hydrogencarbonate ion can be represented by Equations 3 and 4.
Equation (3) OH− (aq) + CO2 (g) → HCO3− (aq)
Equation (4) OH− (aq) + HCO3− (aq) → H2O (l) + CO32− (aq)
Discuss how these equations show the difference between a Lewis base and a Brønsted–Lowry base.
Equation (3):
Equation (4):
Aqueous sodium hydrogencarbonate has a pH of approximately 7 at 298 K.
Sketch a graph of pH against volume when 25.0cm3 of 0.100 mol dm−3 NaOH (aq) is gradually added to 10.0cm3 of 0.0500 mol dm−3 NaHCO3 (aq).
Ammonia is soluble in water and forms an alkaline solution:
NH3 (g) + H2O (l) NH4+ (aq) + HO– (aq)
State the relationship between NH4+ and NH3 in terms of the Brønsted–Lowry theory.
Determine the concentration, in mol dm–3, of the solution formed when 900.0 dm3 of NH3 (g) at 300.0 K and 100.0 kPa, is dissolved in water to form 2.00 dm3 of solution. Use sections 1 and 2 of the data booklet.
Calculate the concentration of hydroxide ions in an ammonia solution with pH = 9.3. Use sections 1 and 2 of the data booklet.
Calculate the concentration, in mol dm–3, of ammonia molecules in the solution with pH = 9.3. Use section 21 of the data booklet.
An aqueous solution containing high concentrations of both NH3 and NH4+ acts as an acid-base buffer solution as a result of the equilibrium:
NH3 (aq) + H+ (aq) NH4+ (aq)
Referring to this equilibrium, outline why adding a small volume of strong acid would leave the pH of the buffer solution almost unchanged.
Magnesium salts form slightly acidic solutions owing to equilibria such as:
Mg2+ (aq) + H2O (l) Mg(OH)+ (aq) + H+ (aq)
Comment on the role of Mg2+ in forming the Mg(OH)+ ion, in acid-base terms.
Mg(OH)+ is a complex ion, but Mg is not regarded as a transition metal. Contrast Mg with manganese, Mn, in terms of one characteristic chemical property of transition metals, other than complex ion formation.
Ethanol and methanoic acid are important industrial products.
Ethanol is used as a fuel.
Write the chemical equation for the complete combustion of ethanol.
Deduce the change in enthalpy, ΔH, in kJ, when 56.00 g of ethanol is burned. Use section 13 in the data booklet.
Oxidation of ethanol with potassium dichromate, K2Cr2O7, can form two different organic products. Determine the names of the organic products and the methods used to isolate them.
Write the equation and name the organic product when ethanol reacts with methanoic acid.
Sketch the titration curve of methanoic acid with sodium hydroxide, showing how you would determine methanoic acid pKa.
Identify an indicator that could be used for the titration in 5(d)(i), using section 22 of the data booklet.
Determine the concentration of methanoic acid in a solution of pH = 4.12. Use section 21 of the data booklet.
Identify if aqueous solutions of the following salts are acidic, basic, or neutral.
This question is about carbon and chlorine compounds.
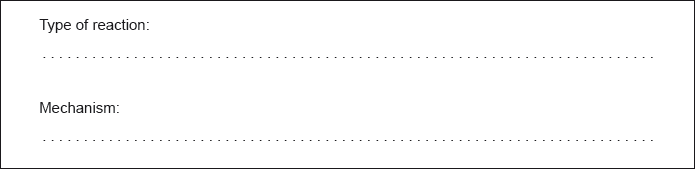
Ethane, , reacts with chlorine in sunlight. State the type of this reaction and the name of the mechanism by which it occurs.
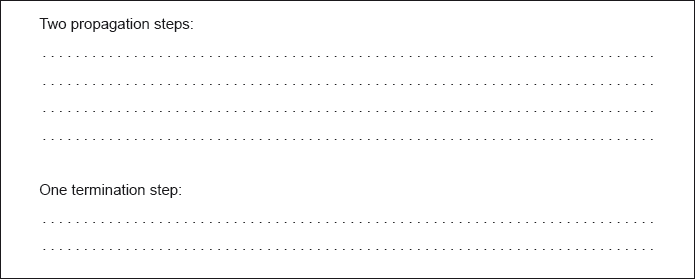
Formulate equations for the two propagation steps and one termination step in the formation of chloroethane from ethane.
Deduce the splitting patterns in the 1H NMR spectrum of C2H5Cl.
Explain why tetramethylsilane (TMS) is often used as a reference standard in 1H NMR.
One possible product, X, of the reaction of ethane with chlorine has the following composition by mass:
carbon: 24.27%, hydrogen: 4.08%, chlorine: 71.65%
Determine the empirical formula of the product.
The mass and 1H NMR spectra of product X are shown below. Deduce, giving your reasons, its structural formula and hence the name of the compound.
When the product X is reacted with NaOH in a hot alcoholic solution, C2H3Cl is formed. State the role of the reactant NaOH other than as a nucleophile.
Chloroethene, , can undergo polymerization. Draw a section of the polymer with three repeating units.
Dinitrogen monoxide, N2O, causes depletion of ozone in the stratosphere.
Different sources of N2O have different ratios of 14N : 15N.
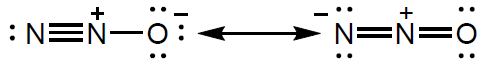
The Lewis (electron dot) structure of the dinitrogen monoxide molecule can be represented as:
Outline why ozone in the stratosphere is important.
Dinitrogen monoxide in the stratosphere is converted to nitrogen monoxide, NO (g).
Write two equations to show how NO (g) catalyses the decomposition of ozone.
State one analytical technique that could be used to determine the ratio of 14N : 15N.
A sample of gas was enriched to contain 2 % by mass of 15N with the remainder being 14N.
Calculate the relative molecular mass of the resulting N2O.
Predict, giving two reasons, how the first ionization energy of 15N compares with that of 14N.
Explain why the first ionization energy of nitrogen is greater than both carbon and oxygen.
Nitrogen and carbon:
Nitrogen and oxygen:
State what the presence of alternative Lewis structures shows about the nature of the bonding in the molecule.
State, giving a reason, the shape of the dinitrogen monoxide molecule.
Deduce the hybridization of the central nitrogen atom in the molecule.
Iron may be extracted from iron (II) sulfide, FeS.
Iron (II) sulfide, FeS, is ionically bonded.
The first step in the extraction of iron from iron (II) sulfide is to roast it in air to form iron (III) oxide and sulfur dioxide.
Outline why metals, like iron, can conduct electricity.
Justify why sulfur is classified as a non-metal by giving two of its chemical properties.
Sketch the first eight successive ionisation energies of sulfur.
Describe the bonding in this type of solid.
State a technique that could be used to determine the crystal structure of the solid compound.
State the full electron configuration of the sulfide ion.
Outline, in terms of their electronic structures, why the ionic radius of the sulfide ion is greater than that of the oxide ion.
Suggest why chemists find it convenient to classify bonding into ionic, covalent and metallic.
Write the equation for this reaction.
Deduce the change in the oxidation state of sulfur.
Suggest why this process might raise environmental concerns.
Explain why the addition of small amounts of carbon to iron makes the metal harder.