
SL Paper 2
Molten sodium chloride can be electrolysed using graphite electrodes.
Draw the essential components of this electrolytic cell and identify the products that form at each electrode.
Product formed at positive electrode (anode):
Product formed at negative electrode (cathode):
State the half-equations for the oxidation and reduction processes and deduce the overall cell reaction, including state symbols.
Oxidation half-equation:
Reduction half-equation:
Overall cell reaction:
Explain why solid sodium chloride does not conduct electricity.
Bonds can be formed in many ways.
The landing module for the Apollo mission used rocket fuel made from a mixture of hydrazine, N2H4, and dinitrogen tetraoxide, N2O4.
N2H4(l) + N2O4(l) → 3N2(g) + 4H2O(g)
State and explain the difference in bond strength between the nitrogen atoms in a hydrazine and nitrogen molecule.
State why hydrazine has a higher boiling point than dinitrogen tetraoxide.
Determine the oxidation state of nitrogen in the two reactants.
Deduce, giving a reason, which species is the reducing agent.
Deduce the Lewis (electron dot) structures of ozone.
Hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}}\), releases oxygen gas, \({{\text{O}}_{\text{2}}}{\text{(g)}}\), as it decomposes according to the equation below.
\[{\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\]
\({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of hydrogen peroxide solution was placed in a boiling tube, and a drop of liquid detergent was added to create a layer of bubbles on the top of the hydrogen peroxide solution as oxygen gas was released. The tube was placed in a water bath at 75 °C and the height of the bubble layer was measured every thirty seconds. A graph was plotted of the height of the bubble layer against time.
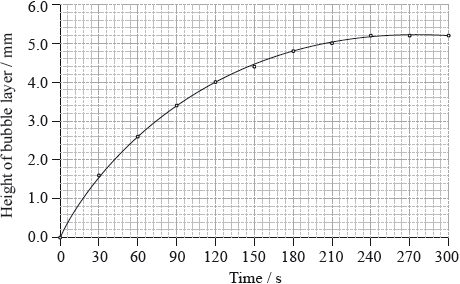
The experiment was repeated using solid manganese(IV) oxide, \({\text{Mn}}{{\text{O}}_{\text{2}}}{\text{(s)}}\), as a catalyst.
The decomposition of hydrogen peroxide to form water and oxygen is a redox reaction.
Explain why the curve reaches a maximum.
Use the graph to calculate the rate of decomposition of hydrogen peroxide at 120 s.
(i) Draw a curve on the graph opposite to show how the height of the bubble layer changes with time when manganese(IV) oxide is present.
(ii) Explain the effect of the catalyst on the rate of decomposition of hydrogen peroxide.
(i) Deduce the oxidation numbers of oxygen present in each of the species below.
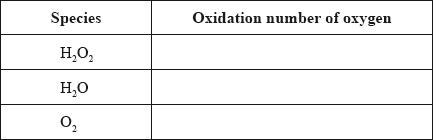
(ii) State two half-equations for the decomposition of hydrogen peroxide.
Oxidation:
Reduction:
Chlorine can be made by reacting concentrated hydrochloric acid with potassium manganate(VII), \({\text{KMn}}{{\text{O}}_{\text{4}}}\).
\[{\text{2KMn}}{{\text{O}}_4}{\text{(aq)}} + {\text{16HCl(aq)}} \to {\text{2MnC}}{{\text{l}}_2}{\text{(aq)}} + {\text{2KCl(aq)}} + {\text{5C}}{{\text{l}}_2}{\text{(aq)}} + {\text{8}}{{\text{H}}_2}{\text{O(aq)}}\]
Define oxidation in terms of electron transfer.
State the oxidation number of manganese in \({\text{KMn}}{{\text{O}}_{\text{4}}}\) and in \({\text{MnC}}{{\text{l}}_{\text{2}}}\).
\({\text{KMn}}{{\text{O}}_{\text{4}}}\)
\({\text{MnC}}{{\text{l}}_{\text{2}}}\)
Deduce which species has been oxidized in this reaction and state the change in oxidation number that it has undergone.
Phosphine (IUPAC name phosphane) is a hydride of phosphorus, with the formula PH3.
(i) Draw a Lewis (electron dot) structure of phosphine.
(ii) Outline whether you expect the bonds in phosphine to be polar or non-polar, giving a brief reason.
(iii) Explain why the phosphine molecule is not planar.
(iv) Phosphine has a much greater molar mass than ammonia. Explain why phosphine has a significantly lower boiling point than ammonia.
Phosphine is usually prepared by heating white phosphorus, one of the allotropes of phosphorus, with concentrated aqueous sodium hydroxide. The equation for the reaction is:
P4 (s) + 3OH− (aq) + 3H2O (l) → PH3 (g) + 3H2PO2− (aq)
(i) Identify one other element that has allotropes and list two of its allotropes.
Element:
Allotrope 1:
Allotrope 2:
(ii) The first reagent is written as P4, not 4P. Describe the difference between P4 and 4P.
(iii) The ion H2PO2− is amphiprotic. Outline what is meant by amphiprotic, giving the formulas of both species it is converted to when it behaves in this manner.
(iv) State the oxidation state of phosphorus in P4 and H2PO2−.
P4:
H2PO2−:
(v) Oxidation is now defined in terms of change of oxidation number. Explore how earlier definitions of oxidation and reduction may have led to conflicting answers for the conversion of P4 to H2PO2− and the way in which the use of oxidation numbers has resolved this.
2.478 g of white phosphorus was used to make phosphine according to the equation:
P4(s) +3OH−(aq)+3H2O(l) → PH3(g)+3H2PO2−(aq)
(i) Calculate the amount, in mol, of white phosphorus used.
(ii) This phosphorus was reacted with 100.0 cm3 of 5.00 mol dm−3 aqueous sodium hydroxide. Deduce, showing your working, which was the limiting reagent.
(iii) Determine the excess amount, in mol, of the other reagent.
(iv) Determine the volume of phosphine, measured in cm3 at standard temperature and pressure, that was produced.
Consider the following equilibrium.
\[\begin{array}{*{20}{l}} {{\text{2S}}{{\text{O}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_3}{\text{(g)}}}&{\Delta {H^\Theta } = - 198{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
State and explain the effect of increasing the temperature on the yield of sulfur trioxide.
State the effect of a catalyst on the value of \({K_{\text{c}}}\).
State and explain the effect of a catalyst on the position of equilibrium.
Define oxidation in terms of oxidation numbers.
Describe using a labelled diagram, the essential components of an electrolytic cell.
Explain why solid sodium chloride does not conduct electricity but molten sodium chloride does.
Molten sodium chloride undergoes electrolysis in an electrolytic cell. For each electrode deduce the half-equation and state whether oxidation or reduction takes place. Deduce the equation of the overall cell reaction including state symbols.
Electrolysis has made it possible to obtain reactive metals such as aluminium from their ores, which has resulted in significant developments in engineering and technology. State one reason why aluminium is preferred to iron in many uses.
Outline two differences between an electrolytic cell and a voltaic cell.
The element antimony, Sb, is usually found in nature as its sulfide ore, stibnite, \({\text{S}}{{\text{b}}_{\text{2}}}{{\text{S}}_{\text{3}}}\). This ore was used two thousand years ago by ancient Egyptian women as a cosmetic to darken their eyes and eyelashes.
One method of extracting antimony from its sulfide ore is to roast the stibnite in air. This forms antimony oxide and sulfur dioxide. The antimony oxide is then reduced by carbon to form the free element.
Deduce the oxidation number of antimony in stibnite.
Deduce one other common oxidation number exhibited by antimony in some of its compounds.
Deduce the chemical equations for these two reactions.
Ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}\), is a weak base.
Iron is more reactive than copper.
Draw the Lewis structure of ammonia and state the shape of the molecule and its bond angles.
The conjugate acid of ammonia is the ammonium ion, \({\text{NH}}_4^ + \). Draw the Lewis structure of the ammonium ion and deduce its shape and bond angles.
Describe two different properties that could be used to distinguish between a \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a strong monoprotic acid and a \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a weak monoprotic acid.
Explain, using the Brønsted-Lowry theory, how water can act either as an acid or a base. In each case identify the conjugate acid or base formed.
Draw a labelled diagram of a voltaic cell made from an \({\text{Fe(s)}}/{\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) half-cell connected to a \({\text{Cu(s)}}/{\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\) half-cell. In your diagram identify the positive electrode (cathode), the negative electrode (anode) and the direction of electron flow in the external circuit.
Deduce the half-equations for the reactions taking place at the positive electrode (cathode) and negative electrode (anode) of this voltaic cell.
Deduce the overall equation for the reaction taking place in the voltaic cell and determine which species acts as the oxidizing agent and which species has been reduced.
Sodium oxide, \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\), is a white solid with a high melting point.
Explain why solid sodium oxide is a non-conductor of electricity.
Molten sodium oxide is a good conductor of electricity. State the half-equation for the reaction occurring at the positive electrode during the electrolysis of molten sodium oxide.
State the acid-base nature of sodium oxide.
State the equation for the reaction of sodium oxide with water.
The data below is from an experiment used to determine the percentage of iron present in a sample of iron ore. This sample was dissolved in acid and all of the iron was converted to \({\text{F}}{{\text{e}}^{{\text{2 + }}}}\). The resulting solution was titrated with a standard solution of potassium manganate(VII), \({\text{KMn}}{{\text{O}}_{\text{4}}}\). This procedure was carried out three times. In acidic solution, \({\text{MnO}}_4^ - \) reacts with \({\text{F}}{{\text{e}}^{2 + }}\) ions to form \({\text{M}}{{\text{n}}^{2 + }}\) and \({\text{F}}{{\text{e}}^{3 + }}\) and the end point is indicated by a slight pink colour.
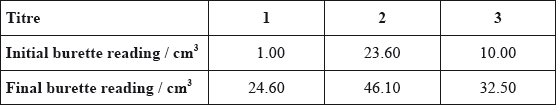

Deduce the balanced redox equation for this reaction in acidic solution.
Identify the reducing agent in the reaction.
Calculate the amount, in moles, of \({\text{MnO}}_4^ - \) used in the titration.
Calculate the amount, in moles, of Fe present in the \(3.682 \times {10^{ - 1}}{\text{ g}}\) sample of iron ore.
Determine the percentage by mass of Fe present in the \(3.682 \times {10^{ - 1}}{\text{ g}}\) sample of iron ore.
Vanadium, another transition metal, has a number of different oxidation states.
Determine the oxidation state of vanadium in each of the following species.
Formulate an equation for the reaction between VO2+(aq) and V2+(aq) in acidic solution to form V3+(aq).
Ethane-1,2-diol, HOCH2CH2OH, has a wide variety of uses including the removal of ice from aircraft and heat transfer in a solar cell.
Ethane-1,2-diol can be formed according to the following reaction.
2CO (g) + 3H2 (g) \( \rightleftharpoons \) HOCH2CH2OH (g)
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) State how increasing the pressure of the reaction mixture at constant temperature will affect the position of equilibrium and the value of Kc.
Position of equilibrium:
Kc:
(iii) Calculate the enthalpy change, ΔHθ, in kJ, for this reaction using section 11 of the data booklet. The bond enthalpy of the carbon–oxygen bond in CO (g) is 1077kJmol-1.
(iv) The enthalpy change, ΔHθ, for the following similar reaction is –233.8 kJ.
2CO(g) + 3H2(g) \( \rightleftharpoons \) HOCH2CH2OH (l)
Deduce why this value differs from your answer to (a)(iii).
Determine the average oxidation state of carbon in ethene and in ethane-1,2-diol.
Ethene:
Ethane-1,2-diol:
Explain why the boiling point of ethane-1,2-diol is significantly greater than that of ethene.
Ethane-1,2-diol can be oxidized first to ethanedioic acid, (COOH)2, and then to carbon dioxide and water. Suggest the reagents to oxidize ethane-1,2-diol.
Brass is a copper containing alloy with many uses. An analysis is carried out to determine the percentage of copper present in three identical samples of brass. The reactions involved in this analysis are shown below.
\[\begin{array}{*{20}{l}} {{\text{Step 1: Cu(s)}} + {\text{2HN}}{{\text{O}}_3}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2N}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}} \\ {{\text{Step 2: 4}}{{\text{I}}^ - }{\text{(aq)}} + {\text{2C}}{{\text{u}}^{2 + }}{\text{(aq)}} \to {\text{2CuI(s)}} + {{\text{I}}_2}{\text{(aq)}}} \\ {{\text{Step 3: }}{{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} \to {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {{\text{S}}_4}{\text{O}}_6^{2 - }{\text{(aq)}}} \end{array}\]
(a) (i) Deduce the change in the oxidation numbers of copper and nitrogen in step 1.
Copper:
Nitrogen:
(ii) Identify the oxidizing agent in step 1.
(b) A student carried out this experiment three times, with three identical small brass nails, and obtained the following results.
\[{\text{Mass of brass}} = 0.456{\text{ g}} \pm 0.001{\text{ g}}\]
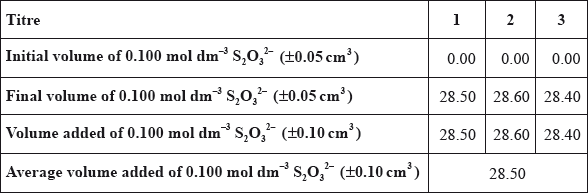
(i) Calculate the average amount, in mol, of \({{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\) added in step 3.
(ii) Calculate the amount, in mol, of copper present in the brass.
(iii) Calculate the mass of copper in the brass.
(iv) Calculate the percentage by mass of copper in the brass.
(v) The manufacturers claim that the sample of brass contains 44.2% copper by mass. Determine the percentage error in the result.
(c) With reference to its metallic structure, describe how brass conducts electricity.
Consider the following three redox reactions.
\[\begin{array}{*{20}{l}} {{\text{Cd(s)}} + {\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} \to {\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} + {\text{Ni(s)}}} \\ {{\text{Ni(s)}} + {\text{2A}}{{\text{g}}^ + }{\text{(aq)}} \to {\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} + {\text{2Ag(s)}}} \\ {{\text{Zn(s)}} + {\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} \to {\text{Z}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{Cd(s)}}} \end{array}\]
(i) Draw an annotated diagram of a voltaic cell composed of a magnesium electrode in \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) magnesium nitrate solution and a silver electrode in \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) silver nitrate solution. State the direction of electron flow on your diagram.
(ii) Deduce half-equations for the oxidation and reduction reactions.
(i) Deduce the order of reactivity of the four metals, cadmium, nickel, silver and zinc and list in order of decreasing reactivity.
(ii) Identify the best oxidizing agent and the best reducing agent.
(i) Solid sodium chloride does not conduct electricity but molten sodium chloride does. Explain this difference.
(ii) Outline what happens in an electrolytic cell during the electrolysis of molten sodium chloride using inert electrodes. Deduce equations for the reactions occurring at each electrode.
(i) A state of equilibrium can exist when a piece of copper metal is placed in a solution of copper(II) sulfate. Outline the characteristics of a chemical system in dynamic equilibrium.
(ii) For an exothermic reaction state how an increase in temperature would affect both \({K_{\text{c}}}\) and the position of equilibrium.
Phosphorus tribromide (\({\text{PB}}{{\text{r}}_{\text{3}}}\)) is used to manufacture alprazolam, a drug used to treat anxiety disorders. Methanal (HCHO) is used as a disinfectant.
Consider the following reaction sequence:
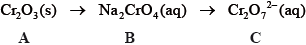
Deduce the balanced chemical equation for the reaction between sodium and sulfur. State the electron arrangements of the reactants and product, and explain whether sulfur is oxidized or reduced.
Describe the acid-base character of the oxides of the period 3 elements, Na to Cl. For the compounds sodium oxide and phosphorus(V) oxide, state the balanced chemical equations for the reaction of each oxide with water.
For each of the species \({\text{PB}}{{\text{r}}_{\text{3}}}\) and HCHO:
• deduce the Lewis structure.
• predict the shape and bond angle.
Explain why \({\text{PB}}{{\text{r}}_{\text{3}}}\) is a polar molecule.
State the name of A.
Describe the redox behaviour of chromium with reference to oxidation numbers in the conversion of B to C.
Define the term oxidizing agent and identify the oxidizing agent in the following
reaction.
\[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {{\text{I}}^ - }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{IO}}_3^ - {\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\]
A hydrocarbon has the empirical formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{7}}}\). When 1.17 g of the compound is heated to 85 °C at a pressure of 101 kPa it occupies a volume of \({\text{400 c}}{{\text{m}}^{\text{3}}}\).
(i) Calculate the molar mass of the compound, showing your working.
(ii) Deduce the molecular formula of the compound.
\({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) exists as three isomers. Identify the structure of the isomer with the lowest boiling point and explain your choice.
Ethanol is a primary alcohol that can be oxidized by acidified potassium dichromate(VI). Distinguish between the reaction conditions needed to produce ethanal and ethanoic acid.
Ethanal:
Ethanoic acid:
Determine the oxidation number of carbon in ethanol and ethanal.
Ethanol:
Ethanal:
Deduce the half-equation for the oxidation of ethanol to ethanal.
Deduce the overall redox equation for the reaction of ethanol to ethanal with acidified potassium dichromate(VI) by combining your answer to part (c) (iii) with the following half-equation:
\[{\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{2 - }{\text{(aq)}} + {\text{14}}{{\text{H}}^ + }{\text{(aq)}} + {\text{6}}{{\text{e}}^ - } \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
Describe two characteristics of a reaction at equilibrium.
Describe how a catalyst increases the rate of a reaction.
State and explain the effect of a catalyst on the position of equilibrium.
Ethanoic acid reacts with ethanol to form the ester ethyl ethanoate.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH(l)}} + {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH(l)?????C}}{{\text{H}}_{\text{3}}}{\text{COOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{(l)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\]
The esterification reaction is exothermic. State the effect of increasing temperature on the value of the equilibrium constant (\({K_{\text{c}}}\)) for this reaction.
In acidic solution, ions containing titanium can react according to the half-equation below.
\[{\text{Ti}}{{\text{O}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \rightleftharpoons {\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}\]
A reactivity series comparing titanium, cadmium and europium is given below.
Least reactive Cd \( < \) Ti \( < \) Eu Most reactive
The half-equations corresponding to these metals are:
\({\text{E}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Eu(s)}}\)
\({\text{T}}{{\text{i}}^{3 + }}{\text{(aq)}} + {\text{3}}{{\text{e}}^ - } \rightleftharpoons {\text{Ti(s)}}\)
\({\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \rightleftharpoons {\text{Cd(s)}}\)
Some students were provided with a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a monobasic acid, HQ, and given the problem of determining whether HQ was a weak acid or a strong acid.
State the initial and final oxidation numbers of titanium and hence deduce whether it is oxidized or reduced in this change.

Considering the above equilibrium, predict, giving a reason, how adding more acid would affect the strength of the \({\text{Ti}}{{\text{O}}^{2 + }}\) ion as an oxidizing agent.
Deduce which of the species would react with titanium metal.
Deduce the balanced equation for this reaction.
Deduce which of the six species is the strongest oxidizing agent.
A voltaic cell can be constructed using cadmium and europium half-cells. State how the two solutions involved should be connected and outline how this connection works.
Define a Brønsted–Lowry acid.
Distinguish between the terms strong acid and weak acid.
Neelu and Charles decided to solve the problem by determining the volume of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution needed to neutralize \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of the acid. Outline whether this was a good choice.
Neelu and Charles decided to compare the volume of sodium hydroxide solution needed with those required by known \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) strong and weak acids. Unfortunately they chose sulfuric acid as the strong acid. Outline why this was an unsuitable choice.
State a suitable choice for both the strong acid and the weak acid.
Strong acid:
Weak acid:
Francisco and Shamiso decided to measure the pH of the initial solution, HQ, and they found that its pH was 3.7. Deduce, giving a reason, the strength (weak or strong) of the acid HQ.
Suggest a method, other than those mentioned above, that could be used to solve the problem and outline how the results would distinguish between a strong acid and a weak acid.
The periodic table shows the relationship between electron arrangement and the properties of elements and is a valuable tool for making predictions in chemistry.
The word redox comes from a combination of the terms reduction and oxidation. Redox reactions affect our daily lives.
The overall reaction that takes place in a voltaic cell is shown below.
\[{\text{Pb(s)}} + {\text{Pb}}{{\text{O}}_{\text{2}}}{\text{(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}} \to {\text{2PbS}}{{\text{O}}_{\text{4}}}{\text{(s)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}}\]
(i) Identify the property used to arrange the elements in the periodic table.
(ii) Outline two reasons why electronegativity increases across period 3 in the periodic table and one reason why noble gases are not assigned electronegativity values.
(i) Define the term first ionization energy of an atom.
(ii) Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
(iii) Explain why sodium conducts electricity but phosphorus does not.
(i) Determine the oxidation number of lead in Pb, \({\text{Pb}}{{\text{O}}_{\text{2}}}\) and \({\text{PbS}}{{\text{O}}_{\text{4}}}\).
(ii) Deduce the oxidation and reduction half-equations taking place at the negative lead electrode (anode) and the positive lead(IV) oxide electrode (cathode). Deduce the oxidizing and reducing agents and state the direction of the electron flow between the electrodes.
(iii) In order to determine the position of three metals in a reactivity series, the metals were placed in different solutions of metal ions. The table below summarizes whether or not a reaction occurred.
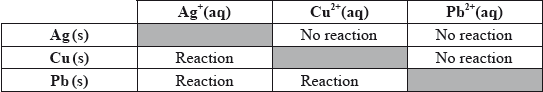
State the equations for the three reactions that take place. Use this information to place the metals Ag, Cu and Pb in a reactivity series, with the strongest reducing agent first, and explain your reasoning.
Ethanedioic acid (oxalic acid), \({{\text{(COOH)}}_{\text{2}}}\), reacts with acidified potassium permanganate solution, \({\text{KMn}}{{\text{O}}_{\text{4}}}\), according to the following equation.
\[{\text{5(COOH}}{{\text{)}}_2}{\text{(aq)}} + {\text{2MnO}}_4^ - {\text{(aq)}} + {\text{6}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{10C}}{{\text{O}}_2}{\text{(g)}} + {\text{2M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{8}}{{\text{H}}_2}{\text{O(l)}}\]
The reaction is a redox reaction.
Define oxidation in terms of electron transfer.
Calculate the change in oxidation numbers of carbon and manganese.
Carbon:
Manganese:
Identify the oxidizing and reducing agents.
Oxidizing agent:
Reducing agent:
Iron tablets are often prescribed to patients. The iron in the tablets is commonly present as iron(II) sulfate, \({\text{FeS}}{{\text{O}}_{\text{4}}}\).
Two students carried out an experiment to determine the percentage by mass of iron in a brand of tablets marketed in Cyprus.
Experimental Procedure:
• The students took five iron tablets and found that the total mass was 1.65 g.
• The five tablets were ground and dissolved in \({\text{100 c}}{{\text{m}}^{\text{3}}}\) dilute sulfuric acid, \({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\). The solution and washings were transferred to a \({\text{250 c}}{{\text{m}}^{\text{3}}}\) volumetric flask and made up to the mark with deionized (distilled) water.
• \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of this \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) solution was transferred using a pipette into a conical flask. Some dilute sulfuric acid was added.
• A titration was then carried out using a \(5.00 \times {10^{ - 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) standard solution of potassium permanganate, \({\text{KMn}}{{\text{O}}_{\text{4}}}{\text{(aq)}}\). The end-point of the titration was indicated by a slight pink colour.
The following results were recorded.
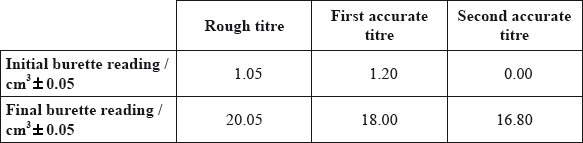
This experiment involves the following redox reaction.
\[{\text{5F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{MnO}}_4^ - {\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{5F}}{{\text{e}}^{3 + }}{\text{(aq)}} + {\text{M}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\]
When the \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) solution was made up in the \({\text{250 c}}{{\text{m}}^{\text{3}}}\) volumetric flask, deionized (distilled) water was added until the bottom of its meniscus corresponded to the graduation mark on the flask. It was noticed that one of the two students measured the volume of the solution from the top of the meniscus instead of from the bottom. State the name of this type of error.
State what is meant by the term precision.
When the students recorded the burette readings, following the titration with KMnO4 (aq),the top of the meniscus was used and not the bottom. Suggest why the students read the top of the meniscus and not the bottom.
Define the term reduction in terms of electrons.
Deduce the oxidation number of manganese in the \({\text{MnO}}_{\text{4}}^ - {\text{(aq)}}\) ion.
Determine the amount, in mol, of \({\text{MnO}}_{\text{4}}^ - {\text{(aq)}}\), used in each accurate titre.
Calculate the amount, in mol, of \({\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) ions in \({\text{250 c}}{{\text{m}}^{\text{3}}}\) of the solution.
Determine the total mass of iron, in g, in the \({\text{250 c}}{{\text{m}}^{\text{3}}}\) solution.
Determine the percentage by mass of iron in the tablets.
One titration was abandoned because a brown precipitate, manganese(IV) oxide, formed. State the chemical formula of this compound.
Bromomethane was used as a pesticide until it was found to be ozone-depleting.
State the equation for the reaction between methane and bromine to form bromomethane.
Explain, using equations, the complete free-radical mechanism for the reaction of methane with bromine, including necessary reaction conditions.
Bromine can be produced by the electrolysis of molten sodium bromide. Deduce the half-equation for the reaction at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
Bromine reacts with aqueous sodium iodide:
\[{\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}} + {\text{2NaI(aq)}} \to {{\text{I}}_{\text{2}}}{\text{(aq)}} + {\text{2NaBr(aq)}}\]
Identify the oxidizing agent in this reaction.
Electrolysis is an important industrial process used to obtain very reactive elements from their common ores.
Molten magnesium chloride can be electrolysed using inert graphite electrodes at 800 °C.
Describe, using a labelled diagram, the essential components of this electrolytic cell.
Molten magnesium chloride can be electrolysed using inert graphite electrodes at 800 °C.
Deduce the half-equations, including state symbols, for the reactions occurring at each electrode. (The melting points of MgCl2 and Mg are 714 °C and 649 °C respectively.)
Positive electrode (anode):
Negative electrode (cathode):
Outline why solid magnesium chloride does not conduct electricity.
Aluminium can also be obtained by electrolysis. Suggest one reason why aluminium is often used instead of iron by engineers.
Both sodium and sodium chloride can conduct electricity.
Compare how electric current passes through sodium and sodium chloride by completing the table below.
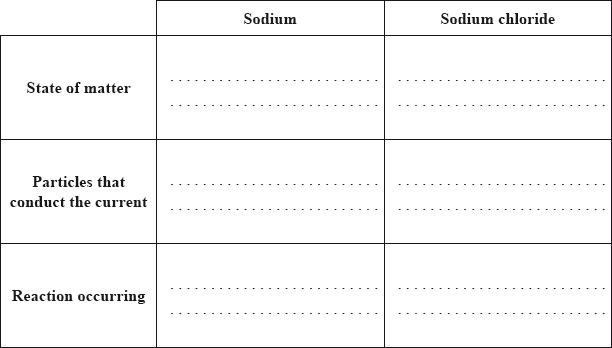
Sodium can be obtained by electrolysis from molten sodium chloride. Describe, using a diagram, the essential components of this electrolytic cell.
Oxidation and reduction can be defined in terms of electron transfer or oxidation numbers.
Alcohols with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) occur as four structural isomers. Three of the isomers can be oxidized with acidified potassium dichromate solution to form compounds with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). The half-equation for the dichromate ion is:
\[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {\text{14}}{{\text{H}}^ + }{\text{(aq)}} + {\text{6}}{{\text{e}}^ - } \rightleftharpoons {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\]
Electrolysis has made it possible to obtain reactive metals from their ores.
A reactivity series can be experimentally determined by adding the metals W, X, Y and Z to solutions of these metal ions. The following reactions were observed:
\({{\text{W}}^{2 + }}{\text{(aq)}} + {\text{X(s)}} \to {\text{W(s)}} + {{\text{X}}^{2 + }}{\text{(aq)}}\)
\({\text{Y(s)}} + {{\text{W}}^{2 + }}{\text{(aq)}} \to {{\text{Y}}^{2 + }}{\text{(aq)}} + {\text{W(s)}}\)
\({{\text{Z}}^{2 + }}{\text{(aq)}} + {\text{W(s)}} \to {\text{Z(s)}} + {{\text{W}}^{2 + }}{\text{(aq)}}\)
\({\text{Y(s)}} + {{\text{X}}^{2 + }}{\text{(aq)}} \to {{\text{Y}}^{2 + }}{\text{(aq)}} + {\text{X(s)}}\)
Define oxidation in terms of electron transfer.
(i) Deduce the oxidation number of chromium in \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{2 - }\).
(ii) Deduce the half-equation for the oxidation of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
(iii) Deduce the overall equation for the redox reaction.
(iv) Two of the isomers with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) can be oxidized further to form compounds with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{{\text{O}}_{\text{2}}}\). Deduce the structural formulas of these two isomers.
(v) One isomer cannot be oxidized by acidified potassium dichromate solution.
Deduce its structural formula, state its name and identify it as a primary, secondary or tertiary alcohol.
Name:
Alcohol:
(vi) All isomers of the alcohol \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\) undergo complete combustion. State an equation for the complete combustion of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\).
(i) Draw a labelled electrolytic cell for the electrolysis of molten potassium bromide, KBr. Include the direction of electron flow, the positive electrode (anode) and the negative electrode (cathode), the location of oxidation and reduction, and the electrolyte.
(ii) Deduce a half-equation for the reaction that occurs at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
(iii) Describe how current is conducted in a molten electrolyte.
(i) Deduce the order of reactivity of these four metals, from the least to the most reactive.
(ii) A voltaic cell is made by connecting a half-cell of X in \({\text{XC}}{{\text{l}}_{\text{2}}}{\text{(aq)}}\) to a half-cell of Z in \({\text{ZC}}{{\text{l}}_{\text{2}}}{\text{(aq)}}\). Deduce the overall equation for the reaction taking place when the cell is operating.
The Haber process enables the large-scale production of ammonia needed to make fertilizers.
The equation for the Haber process is given below.
\[{{\text{N}}_2}({\text{g)}} + 3{{\text{H}}_2}({\text{g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_3}({\text{g)}}\]
The percentage of ammonia in the equilibrium mixture varies with temperature.
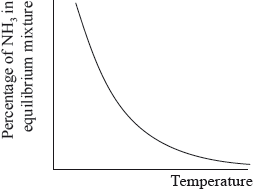
Fertilizers may cause health problems for babies because nitrates can change into nitrites in water used for drinking.
A student decided to investigate the reactions of the two acids with separate samples of \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.
(i) Use the graph to deduce whether the forward reaction is exothermic or endothermic and explain your choice.
(ii) State and explain the effect of increasing the pressure on the yield of ammonia.
(iii) Explain the effect of increasing the temperature on the rate of reaction.
(i) Define oxidation in terms of oxidation numbers.
(ii) Deduce the oxidation states of nitrogen in the nitrate, \({\text{NO}}_{\text{3}}^ - \), and nitrite, \({\text{NO}}_{\text{2}}^ - \), ions.
The nitrite ion is present in nitrous acid, HNO2, which is a weak acid. The nitrate ion is present in nitric acid, HNO3, which is a strong acid. Distinguish between the terms strong and weak acid and state the equations used to show the dissociation of each acid in aqueous solution.
A small piece of magnesium ribbon is added to solutions of nitric and nitrous acid of the same concentration at the same temperature. Describe two observations that would allow you to distinguish between the two acids.
(i) Calculate the volume of the sodium hydroxide solution required to react exactly with a \({\text{15.0 c}}{{\text{m}}^{\text{3}}}\) solution of \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) nitric acid.
(ii) The following hypothesis was suggested by the student: “Since nitrous acid is a weak acid it will react with a smaller volume of the \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.” Comment on whether or not this is a valid hypothesis.
The graph below shows how the conductivity of the two acids changes with concentration.
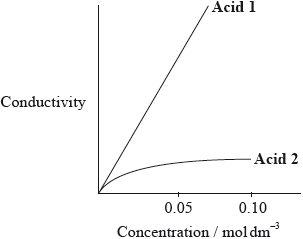
Identify Acid 1 and explain your choice.
Nitric acid reacts with silver in a redox reaction.
__ \({\text{Ag(s)}} + \) __ \({\text{NO}}_3^ - {\text{(aq)}} + \) ___ \( \to \) ___\({\text{A}}{{\text{g}}^ + }{\text{(aq)}} + \) __ \({\text{NO(g)}} + \) ____
Using oxidation numbers, deduce the complete balanced equation for the reaction showing all the reactants and products.
Iron rusts in the presence of oxygen and water. Rusting is a redox process involving several steps that produces hydrated iron(III) oxide, \({\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}} \bullet {\text{n}}{{\text{H}}_{\text{2}}}{\text{O}}\), as the final product.
The half-equations involved for the first step of rusting are given below.
Half-equation 1: \({\text{Fe(s)}} \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\)
Half-equation 2: \({{\text{O}}_{\text{2}}}{\text{(aq)}} + {\text{4}}{{\text{e}}^ - } + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\)
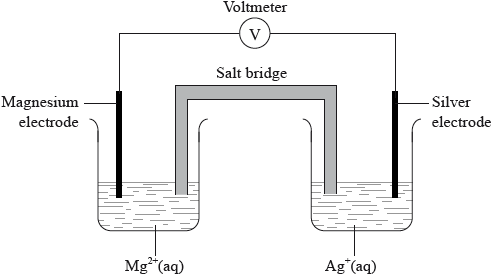
A voltaic cell is made from a half-cell containing a magnesium electrode in a solution of magnesium nitrate and a half-cell containing a silver electrode in a solution of silver(I) nitrate.
Hydrogen peroxide decomposes according to the equation below.
\[{\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\]
The rate of the decomposition can be monitored by measuring the volume of oxygen gas released. The graph shows the results obtained when a solution of hydrogen peroxide decomposed in the presence of a CuO catalyst.
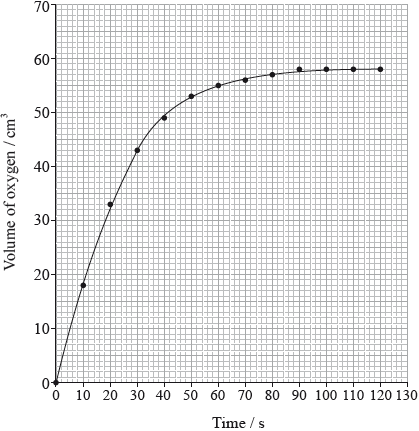
(i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason for your answer.
(ii) Identify the oxidation number of each atom in the three species in half-equation 2.
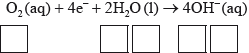
(iii) Deduce the overall redox equation for the first step of rusting by combining half-equations 1 and 2.
(iv) Identify the reducing agent in the redox equation in part (iii).
The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very small concentrations. Explain, in terms of intermolecular forces, why oxygen is not very soluble in water.
(i) Given that magnesium is more reactive than silver, deduce the half-equations for the reactions occurring at each electrode, including state symbols.
Negative electrode (anode):
Positive electrode (cathode):
(ii) Outline one function of the salt bridge.
(i) State the property that determines the order in which elements are arranged in the periodic table.
(ii) State the relationship between the electron arrangement of an element and its group and period in the periodic table.
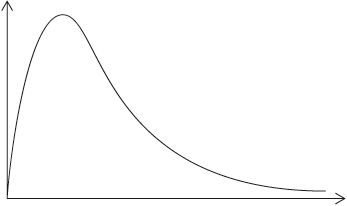
(i) The experiment is repeated with the same amount of a more effective catalyst, \({\text{Mn}}{{\text{O}}_{\text{2}}}\), under the same conditions and using the same concentration and volume of hydrogen peroxide. On the graph above, sketch the curve you would expect.
(ii) Outline how the initial rate of reaction can be found from the graph.
(iii) Outline a different experimental procedure that can be used to monitor the decomposition rate of hydrogen peroxide.
(iv) A Maxwell–Boltzmann energy distribution curve is drawn below. Label both axes and explain, by annotating the graph, how catalysts increase the rate of reaction.
Group 7 of the periodic table contains a number of reactive elements such as chlorine, bromine and iodine.
Bleaches in which chlorine is the active ingredient are the most common, although some environmental groups have concerns about their use. In aqueous chlorine the equilibrium below produces chloric(I) acid (hypochlorous acid), HOCl, the active bleach.
\[{\text{C}}{{\text{l}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HOCl (aq)}} + {{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\]
Aqueous sodium chlorate(I), NaOCl, the most common active ingredient in chlorine based bleaches, oxidizes coloured materials to colourless products while being reduced to the chloride ion. It will also oxidize sulfur dioxide to the sulfate ion.
(i) Describe the colour change that occurs when aqueous chlorine is added to aqueous sodium bromide.
(ii) Outline, with the help of a chemical equation, why this reaction occurs.
The colour change in the reaction between aqueous chlorine and aqueous sodium iodide is very similar, but it differs with an excess of aqueous chlorine. Describe the appearance of the reaction mixture when excess aqueous chlorine has been added to aqueous sodium iodide.
Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is indicated in the equation above.
State a balanced equation for the reaction of chloric(I) acid with water.
Outline, in terms of the equilibrium above, why it is dangerous to use an acidic toilet cleaner in combination with this kind of bleach.
Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
Draw the Lewis (electron dot) structure of chloric(I) acid.
Predict the H–O–Cl bond angle in this molecule and explain this in terms of the valence shell electron pair repulsion (VSEPR) theory.
(i) Deduce the coefficients required to balance the half-equations given below.
___ \({\text{Cl}}{{\text{O}}^ - } + \) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({{\text{H}}_2}{\text{O}} + \) ___ \({\text{C}}{{\text{l}}^ - }\)
___ \({\text{SO}}_4^{2 - }\) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({\text{S}}{{\text{O}}_2} + \) ___ \({{\text{H}}_2}{\text{O}}\)
(ii) State the initial and final oxidation numbers of both chlorine and sulfur in the equations in part (i).

(iii) Use the half-equations to deduce the balanced equation for the reaction between the chlorate(I) ion and sulfur dioxide.
The boiling points of the isomers of pentane, \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\), shown are 10, 28 and 36 °C, but not necessarily in that order.
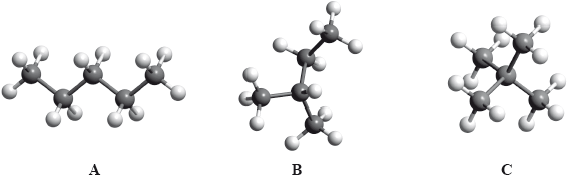
Identify the boiling points for each of the isomers A, B and C and state a reason for your answer.

State the IUPAC names of isomers B and C.
B:
C:
Both \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) and \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{OH}}\) can be used as fuels. Predict which compound would release a greater amount of heat per gram when it undergoes complete combustion. Suggest two reasons to support your prediction.
In many cities around the world, public transport vehicles use diesel, a liquid hydrocarbon fuel, which often contains sulfur impurities and undergoes incomplete combustion. All public transport vehicles in New Delhi, India, have been converted to use compressed natural gas (CNG) as fuel. Suggest two ways in which this improves air quality, giving a reason for your answer.
An acidic sample of a waste solution containing Sn2+(aq) reacted completely with K2Cr2O7 solution to form Sn4+(aq).
State the oxidation half-equation.
Deduce the overall redox equation for the reaction between acidic Sn2+(aq) and Cr2O72–(aq), using section 24 of the data booklet.
Calculate the percentage uncertainty for the mass of K2Cr2O7(s) from the given data.
The sample of K2Cr2O7(s) in (i) was dissolved in distilled water to form 0.100 dm3 solution. Calculate its molar concentration.
10.0 cm3 of the waste sample required 13.24 cm3 of the K2Cr2O7 solution. Calculate the molar concentration of Sn2+(aq) in the waste sample.
Arsenic and nitrogen play a significant role in environmental chemistry. Arsenous acid, H3AsO3, can be found in oxygen-poor (anaerobic) water, and nitrogen-containing fertilizers can contaminate water.
Nitric acid, HNO3, is strong and nitrous acid, HNO2, is weak.
(i) Define oxidation and reduction in terms of electron loss or gain.
Oxidation:
Reduction:
(ii) Deduce the oxidation numbers of arsenic and nitrogen in each of the following species.
\({\text{A}}{{\text{s}}_{\text{2}}}{{\text{O}}_{\text{3}}}\):
\({\text{NO}}_3^ - \):
\({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{3}}}\):
\({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{3}}}\):
(iii) Distinguish between the terms oxidizing agent and reducing agent.
(iv) In the removal of arsenic from contaminated groundwater, \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{3}}}\) is often first oxidized to arsenic acid, \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{4}}}\).
The following unbalanced redox reaction shows another method of forming \({{\text{H}}_{\text{3}}}{\text{As}}{{\text{O}}_{\text{4}}}\).
\[{\text{A}}{{\text{s}}_2}{{\text{O}}_3}{\text{(s)}} + {\text{NO}}_3^ - {\text{(aq)}} \to {{\text{H}}_3}{\text{As}}{{\text{O}}_4}{\text{(aq)}} + {{\text{N}}_2}{{\text{O}}_3}{\text{(aq)}}\]
Deduce the balanced redox equation in acid, and then identify both the oxidizing and reducing agents.
Define an acid according to the Brønsted–Lowry and Lewis theories.
Brønsted–Lowry theory:
Lewis theory:
The Lewis (electron dot) structure of nitrous acid is given below.
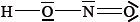
Identify which nitrogen-oxygen bond is the shorter.
Deduce the approximate value of the hydrogen-oxygen-nitrogen bond angle in nitrous acid and explain your answer.
Distinguish between a strong acid and a weak acid in terms of their dissociation in aqueous solution.
Ammonia, NH3, is a weak base. Deduce the Lewis (electron dot) structure of NH3. State the name of the shape of the molecule and explain why NH3 is a polar molecule.
When lime was added to a sample of soil, the pH changed from 5 to 7. Calculate the factor by which the hydrogen ion concentration changes.
One common nitrogen-containing fertilizer is ammonium sulfate. State its chemical formula.
The rate of the acid-catalysed iodination of propanone can be followed by measuring how the concentration of iodine changes with time.
I2(aq) + CH3COCH3(aq) → CH3COCH2I(aq) + H+(aq) + I−(aq)
Suggest how the change of iodine concentration could be followed.
A student produced these results with [H+] = 0.15 mol\(\,\)dm−3. Propanone and acid were in excess and iodine was the limiting reagent.
Determine the relative rate of reaction when [H+] = 0.15 mol\(\,\)dm−3.
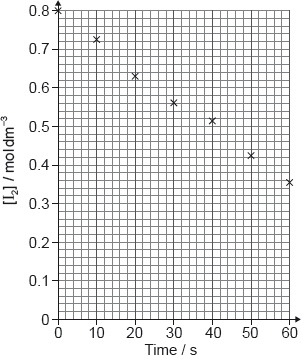
The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
State and explain the relationship between the rate of reaction and the concentration of acid.
The diagram shows an incomplete voltaic cell with a light bulb in the circuit.
Identify the missing component of the cell and its function.
Deduce the half-equations for the reaction at each electrode when current flows.
Annotate the diagram with the location and direction of electron movement when current flows.
The emission spectrum of an element can be used to identify it.
Elements show trends in their physical properties across the periodic table.
Draw the first four energy levels of a hydrogen atom on the axis, labelling n = 1, 2, 3 and 4.
Draw the lines, on your diagram, that represent the electron transitions to n = 2 in the emission spectrum.
Outline why atomic radius decreases across period 3, sodium to chlorine.
Outline why the ionic radius of K+ is smaller than that of Cl−.
Copper is widely used as an electrical conductor.
Draw arrows in the boxes to represent the electronic configuration of copper in the 4s and 3d orbitals.
Impure copper can be purified by electrolysis. In the electrolytic cell, impure copper is the anode (positive electrode), pure copper is the cathode (negative electrode) and the electrolyte is copper(II) sulfate solution.
Formulate the half-equation at each electrode.
Outline where and in which direction the electrons flow during electrolysis.
Magnesium is a group 2 metal which exists as a number of isotopes and forms many compounds.
State the nuclear symbol notation, \({}_Z^AX\), for magnesium-26.
Mass spectroscopic analysis of a sample of magnesium gave the following results:
Calculate the relative atomic mass, Ar, of this sample of magnesium to two decimal places.
Magnesium burns in air to form a white compound, magnesium oxide. Formulate an equation for the reaction of magnesium oxide with water.
Describe the trend in acid-base properties of the oxides of period 3, sodium to chlorine.
In addition to magnesium oxide, magnesium forms another compound when burned in air. Suggest the formula of this compound
Describe the structure and bonding in solid magnesium oxide.
Magnesium chloride can be electrolysed.
Deduce the half-equations for the reactions at each electrode when molten magnesium chloride is electrolysed, showing the state symbols of the products. The melting points of magnesium and magnesium chloride are 922 K and 987 K respectively.
Anode (positive electrode):
Cathode (negative electrode):
Trends in physical and chemical properties are useful to chemists.
The Activity series lists the metal in order of reactivity.
Explain the general increasing trend in the first ionization energies of the period 3 elements, Na to Ar.
Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
State an equation for the reaction of phosphorus (V) oxide, P4O10 (s), with water.
Describe the emission spectrum of hydrogen.
Identify the strongest reducing agent in the given list.
A voltaic cell is made up of a Mn2+/Mn half-cell and a Ni2+/Ni half-cell.
Deduce the equation for the cell reaction.
The voltaic cell stated in part (ii) is partially shown below.
Draw and label the connections needed to show the direction of electron movement and ion flow between the two half-cells.