
HL Paper 3
In an acidic soil, nitrate ions may undergo reduction to form ammonium ions. Deduce a half-equation for the reaction.
Enzymes are catalysts that increase the rate of all biochemical reactions, including those involved in respiration.
Cytochrome oxidase is a complex enzyme that catalyses the reduction of oxygen in the final stage of aerobic respiration. This enzyme is inhibited both by nitrogen(II) oxide, NO, and separately by cyanide ions, \({\text{C}}{{\text{N}}^ - }\). It has been suggested that NO acts competitively while \({\text{C}}{{\text{N}}^ - }\) acts non-competitively in inhibiting the enzyme. Experiments were carried out to test this hypothesis.
The graph below shows the effect of substrate concentration on the rate of the reaction in the absence of an inhibitor. Draw and label the results of the two experiments showing how the rate of the reaction changes in the presence of NO and in the presence of \({\text{C}}{{\text{N}}^ - }\), if the hypothesis is correct.
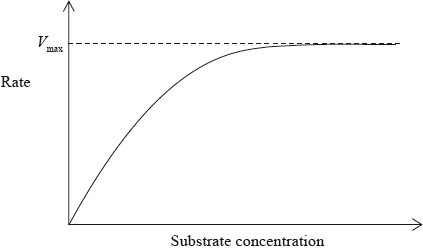
Suggest a reason why it is more likely that NO, rather than \({\text{C}}{{\text{N}}^ - }\), acts competitively.
The reducing agent in the cytochrome oxidase reaction is a species that can be denoted as \({\text{X}}{{\text{H}}_{\text{2}}}\) in the reduced form. Using this notation, deduce an equation for the reaction of \({\text{X}}{{\text{H}}_{\text{2}}}\) and \({{\text{O}}_{\text{2}}}\), and outline, using oxidation numbers, why it is a redox reaction.