
SL Paper 3
Students were asked to investigate how a change in concentration of hydrochloric acid, HCl, affects the initial rate of its reaction with marble chips, CaCO3.
They decided to measure how long the reaction took to complete when similar chips were added to 50.0 cm3 of 1.00 mol dm−3 acid and 50.0 cm3 of 2.00 mol dm−3 acid.
Two methods were proposed:
(1) using small chips, keeping the acid in excess, and recording the time taken for the solid to disappear
(2) using large chips, keeping the marble in excess, and recording the time taken for bubbles to stop forming.
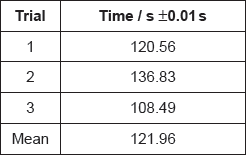
A group recorded the following results with 1.00 mol dm−3 hydrochloric acid:
Annotate the balanced equation below with state symbols.
CaCO3(__) + 2HCl(__) → CaCl2(__) + CO2(__) + H2O(__)
Neither method actually gives the initial rate. Outline a method that would allow the initial rate to be determined.
Deduce, giving a reason, which of the two methods would be least affected by the chips not having exactly the same mass when used with the different concentrations of acid.
State a factor, that has a significant effect on reaction rate, which could vary between marble chips of exactly the same mass.
Justify why it is inappropriate to record the uncertainty of the mean as ±0.01 s.
If doubling the concentration doubles the reaction rate, suggest the mean time you would expect for the reaction with 2.00 mol dm−3 hydrochloric acid.
Another student, working alone, always dropped the marble chips into the acid and then picked up the stopwatch to start it. State, giving a reason, whether this introduced a random or systematic error.
Penicillins and aspirin are important medicines.
Describe how penicillin combats bacterial infections.
State how penicillins may be modified to increase their effectiveness.
State the type of reaction used to synthesize aspirin from salicylic acid.
Explain why aspirin is not stored in a hot, humid location.
Fuel cells and rechargeable batteries are both convenient ways of providing portable electric power.
Compare fuel cells and rechargeable batteries giving one similarity and one difference.
Similarity:
Difference:
One common type of rechargeable cell is the nickel–cadmium (NiCad) battery. For each terminal of this battery state the initial and final oxidation number of the element when the cell is delivering a current. Hence deduce which electrode is acting as the anode and which the cathode.

A common type of fuel cell uses hydrogen and oxygen with an acidic electrolyte. State the half-equations for the reactions at the two electrodes.
Positive electrode:
Negative electrode:
The electrodes of fuel cells and rechargeable batteries have a feature in common with heterogeneous catalysts. Identify this feature and state why it is important for them to work efficiently.
Antacids react with hydrochloric acid in the stomach to relieve indigestion. A student investigated different brands of antacid to see which caused the largest increase in pH in a given time. She added the antacids to hydrochloric acid, and recorded the change in pH over five minutes.
State an equation for the reaction of magnesium hydroxide with hydrochloric acid.
Suggest two variables, besides the time of reaction, which the student should have controlled in the experiment to ensure a fair comparison of the antacids.
Calculate the uncertainty in the change in pH.
The student concluded that antacid B was the most effective, followed by A then C and finally D. Discuss two arguments that reduce the validity of the conclusion.
Palmitic acid has a molar mass of 256.5 g mol−1.
The apparatus in the diagram measures the surface pressure created by palmitic acid molecules on the surface of water. This pressure is caused by palmitic acid molecules colliding with the fixed barrier. The pressure increases as the area, A, available to the palmitic acid is reduced by the movable barrier.
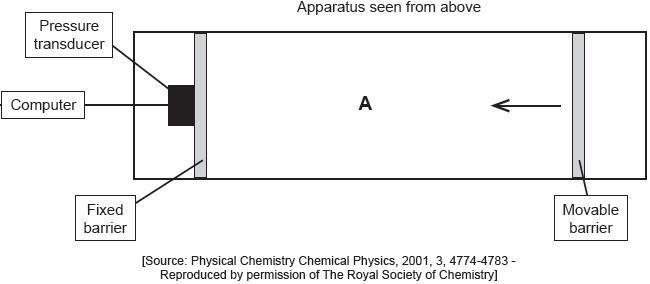
When a drop of a solution of palmitic acid in a volatile solvent is placed between the barriers, the solvent evaporates leaving a surface layer. The graph of pressure against area was obtained as the area A was reduced.
Part of this molecule is hydrophilic (bonds readily to water) and part hydrophobic (does not bond readily to water). Draw a circle around all of the hydrophilic part of the molecule.
When a small amount of palmitic acid is placed in water it disperses to form a layer on the surface that is only one molecule thick. Explain, in terms of intermolecular forces, why this occurs.
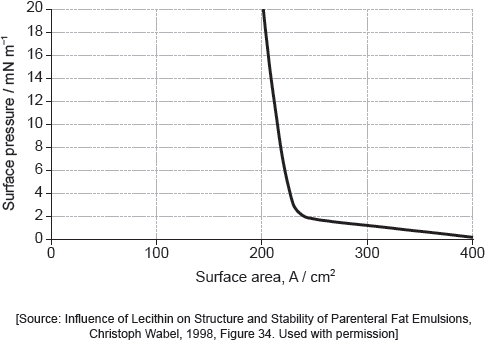
Suggest why there is a small increase in the surface pressure as the area is reduced to about 240 cm2, but a much faster increase when it is further reduced.
The solution of palmitic acid had a concentration of 0.0034 mol dm−3. Calculate the number of molecules of palmitic acid present in the 0.050 cm3 drop, using section 2 of the data booklet.
Assuming the sudden change in gradient occurs at 240 cm2, calculate the area, in cm2, that a single molecule of palmitic acid occupies on surface of the water.
If you did not obtain an answer for (b)(ii) use a value of 8.2 × 1016, but this is not the correct answer.