
HL Paper 2
Nitrogen monoxide reacts at 1280 °C with hydrogen to form nitrogen and water. All reactants and products are in the gaseous phase.
The gas-phase decomposition of dinitrogen monoxide is considered to occur in two steps.
\[\begin{array}{*{20}{l}} {{\text{Step 1:}}}&{{{\text{N}}_2}{\text{O(g)}}\xrightarrow{{{k_1}}}{{\text{N}}_2}({\text{g)}} + {\text{O(g)}}} \\ {{\text{Step 2:}}}&{{{\text{N}}_2}{\text{O(g)}} + {\text{O(g)}}\xrightarrow{{{k_2}}}{{\text{N}}_2}({\text{g)}} + {{\text{O}}_2}{\text{(g)}}} \end{array}\]
The experimental rate expression for this reaction is rate \( = k{\text{[}}{{\text{N}}_2}{\text{O]}}\).
The conversion of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{NC}}\) into \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CN}}\) is an exothermic reaction which can be represented as follows.
\({\text{C}}{{\text{H}}_{\text{3}}}–{\text{N}}\)\( \equiv \)\({\text{C}} \to {\text{transition state}} \to {\text{C}}{{\text{H}}_{\text{3}}}–{\text{C}}\)\( \equiv \)\({\text{N}}\)
This reaction was carried out at different temperatures and a value of the rate constant, \(k\), was obtained for each temperature. A graph of \(\ln k\) against \(1/T\) is shown below.
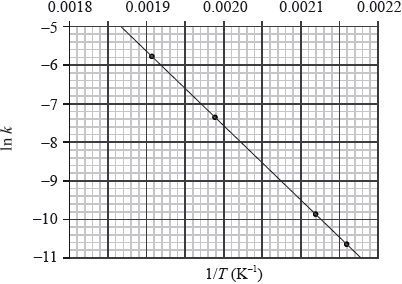
Define the term rate of reaction.
State an equation for the reaction of magnesium carbonate with dilute hydrochloric acid.
The rate of this reaction in (a) (ii), can be studied by measuring the volume of gas collected over a period of time. Sketch a graph which shows how the volume of gas collected changes with time.
The experiment is repeated using a sample of hydrochloric acid with double the volume, but half the concentration of the original acid. Draw a second line on the graph you sketched in part (a) (iii) to show the results in this experiment. Explain why this line is different from the original line.
The kinetics of the reaction were studied at this temperature. The table shows the initial rate of reaction for different concentrations of each reactant.
Deduce the order of the reaction with respect to NO and \({{\text{H}}_2}\), and explain your reasoning.
Deduce the rate expression for the reaction.
Determine the value of the rate constant for the reaction from Experiment 3 and state its units.
Identify the rate-determining step.
Identify the intermediate involved in the reaction.
Define the term activation energy, \({E_{\text{a}}}\).
Construct the enthalpy level diagram and label the activation energy, \({E_{\text{a}}}\), the enthalpy change, \(\Delta H\), and the position of the transition state.
Describe qualitatively the relationship between the rate constant, \(k\), and the temperature, \(T\).
Calculate the activation energy, \({E_{\text{a}}}\), for the reaction, using Table 1 of the Data Booklet.
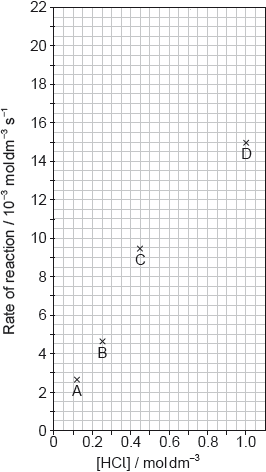
Calcium carbonate reacts with hydrochloric acid.
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
The results of a series of experiments in which the concentration of HCl was varied are shown below.
Outline two ways in which the progress of the reaction can be monitored. No practical details are required.
Suggest why point D is so far out of line assuming human error is not the cause.
Draw the best fit line for the reaction excluding point D.
Suggest the relationship that points A, B and C show between the concentration of the acid and the rate of reaction.
Deduce the rate expression for the reaction.
Calculate the rate constant of the reaction, stating its units.
Predict from your line of best fit the rate of reaction when the concentration of HCl is 1.00 mol dm−3.
Describe how the activation energy of this reaction could be determined.
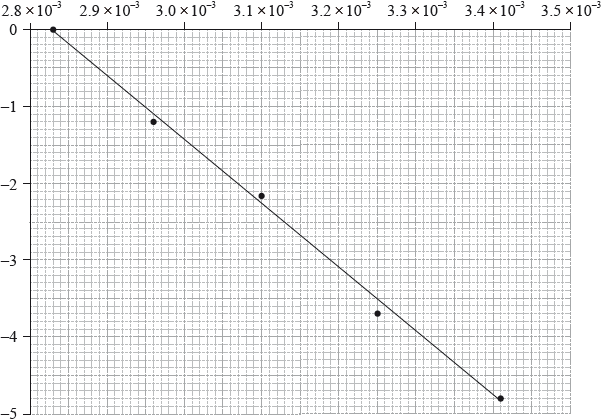
To determine the activation energy of a reaction, the rate of reaction was measured at different temperatures. The rate constant, \(k\), was determined and \(\ln k\) was plotted against the inverse of the temperature in Kelvin, \({T^{ - 1}}\). The following graph was obtained.
Define the term activation energy, \({E_{\text{a}}}\).
Use the graph on page 8 to determine the value of the activation energy, \({E_{\text{a}}}\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
On the graph on page 8, sketch the line you would expect if a catalyst is added to the reactants.
Consider the following reaction studied at 263 K.
\[{\text{2NO(g)}} + {\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2NOCl(g)}}\]
It was found that the forward reaction is first order with respect to \({\rm{C}}{{\rm{l}}_2}\) and second order with respect to NO. The reverse reaction is second order with respect to NOCl.
Consider the following equilibrium reaction.
\[\begin{array}{*{20}{c}} {{\text{C}}{{\text{l}}_2}({\text{g)}} + {\text{S}}{{\text{O}}_2}({\text{g)}} \rightleftharpoons {\text{S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}({\text{g)}}}&{\Delta {H^\Theta } = - 84.5{\text{ kJ}}} \end{array}\]
In a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) closed container, at 375 °C, \({\text{8.60}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\) of \({\text{S}}{{\text{O}}_{\text{2}}}\) and \({\text{8.60}} \times {\text{1}}{{\text{0}}^{ - 3}}{\text{ mol}}\) of \({\text{C}}{{\text{l}}_{\text{2}}}\) were introduced. At equilibrium, \({\text{7.65}} \times {\text{1}}{{\text{0}}^{ - 4}}{\text{ mol}}\) of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) was formed.
State the rate expression for the forward reaction.
Predict the effect on the rate of the forward reaction and on the rate constant if the concentration of NO is halved.
1.0 mol of \({\rm{C}}{{\rm{l}}_2}\) and 1.0 mol of NO are mixed in a closed container at constant temperature. Sketch a graph to show how the concentration of NO and NOCl change with time until after equilibrium has been reached. Identify the point on the graph where equilibrium is established.
Consider the following reaction.
\[{\text{N}}{{\text{O}}_2}{\text{(g)}} + {\text{CO(g)}} \to {\text{NO(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\]
Possible reaction mechanisms are:
\(\begin{array}{*{20}{l}} {{\text{Above 775 K:}}}&{{\text{N}}{{\text{O}}_2} + {\text{CO}} \to {\text{NO}} + {\text{C}}{{\text{O}}_{\text{2}}}}&{{\text{slow}}} \\ {{\text{Below 775 K:}}}&{{\text{2N}}{{\text{O}}_2} \to {\text{NO}} + {\text{N}}{{\text{O}}_{\text{3}}}}&{{\text{slow}}} \\ {}&{{\text{N}}{{\text{O}}_3} + {\text{CO}} \to {\text{N}}{{\text{O}}_2} + {\text{C}}{{\text{O}}_2}}&{{\text{fast}}} \end{array}\)
Based on the mechanisms, deduce the rate expressions above and below 775 K.
State two situations when the rate of a chemical reaction is equal to the rate constant.
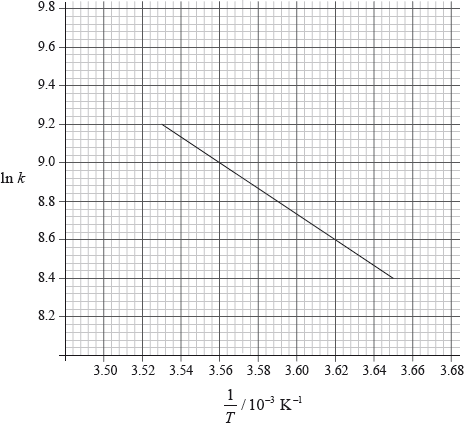
Consider the following graph of \(\ln k\) against \(\frac{1}{T}\) for the first order decomposition of \({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}\) into \({\text{N}}{{\text{O}}_{\text{2}}}\). Determine the activation energy in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) for this reaction.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
Determine the value of the equilibrium constant, \({K_{\text{c}}}\).
If the temperature of the reaction is changed to 300 °C, predict, stating a reason in each case, whether the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) and the value of \({K_{\text{c}}}\) will increase or decrease.
If the volume of the container is changed to \({\text{1.50 d}}{{\text{m}}^{\text{3}}}\), predict, stating a reason in each case, how this will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}\) and the value of \({K_{\text{c}}}\).
Suggest, stating a reason, how the addition of a catalyst at constant pressure and temperature will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
A group of students investigated the rate of the reaction between aqueous sodium thiosulfate and hydrochloric acid according to the equation below.
\[{\text{N}}{{\text{a}}_2}{{\text{S}}_2}{{\text{O}}_3}{\text{(aq)}} + {\text{2HCl(aq)}} \to {\text{2NaCl(aq)}} + {\text{S}}{{\text{O}}_2}{\text{(g)}} + {\text{S(s)}} + {{\text{H}}_2}{\text{O(l )}}\]
The two reagents were rapidly mixed together in a beaker and placed over a mark on a piece of paper. The time taken for the precipitate of sulfur to obscure the mark when viewed through the reaction mixture was recorded.
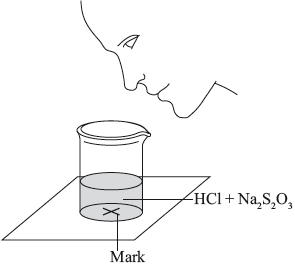
Initially they measured out \({\text{10.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.500 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid and then added \({\text{40.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.0200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium thiosulfate. The mark on the paper was obscured 47 seconds after the solutions were mixed.
One proposed mechanism for this reaction is:
\({{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}} \rightleftharpoons {\text{H}}{{\text{S}}_2}{\text{O}}_3^ - {\text{(aq)}}\) Fast
\({\text{H}}{{\text{S}}_2}{\text{O}}_3^ - {\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}} \to {\text{S}}{{\text{O}}_2}{\text{(g)}} + {\text{S(s)}} + {{\text{H}}_2}{\text{O(l)}}\) Slow
The teacher asked the students to devise another technique to measure the rate of this reaction.
Another group suggested collecting the sulfur dioxide and drawing a graph of the volume of gas against time.
(i) State the volumes of the liquids that should be mixed.
(ii) State why it is important that the students use a similar beaker for both reactions.
(iii) If the reaction were first order with respect to the thiosulfate ion, predict the time it would take for the mark on the paper to be obscured when the concentration of sodium thiosulfate solution is halved.
(i) Deduce the rate expression of this mechanism.
(ii) The results of an experiment investigating the effect of the concentration of hydrochloric acid on the rate, while keeping the concentration of thiosulfate at the original value, are given in the table below.
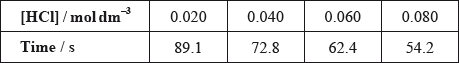
On the axes provided, draw an appropriate graph to investigate the order of the reaction with respect to hydrochloric acid.
(iii) Identify two ways in which these data do not support the rate expression deduced in part (i).
(i) Sketch and label, indicating an approximate activation energy, the Maxwell–Boltzmann energy distribution curves for two temperatures, \({T_1}\) and \(T2{\text{ }}({T_2} > {T_1})\), at which the rate of reaction would be significantly different.

(ii) Explain why increasing the temperature of the reaction mixture would significantly increase the rate of the reaction.
(i) One group suggested recording how long it takes for the pH of the solution to change by one unit. Calculate the initial pH of the original reaction mixture.
(ii) Deduce the percentage of hydrochloric acid that would have to be used up for the pH to change by one unit.
Calculate the volume of sulfur dioxide, in \({\text{c}}{{\text{m}}^{\text{3}}}\), that the original reaction mixture would produce if it were collected at \(1.00 \times {10^5}{\text{ Pa}}\) and 300 K.
Sulfur dioxide, a major cause of acid rain, is quite soluble in water and the equilibrium shown below is established.
\({\text{S}}{{\text{O}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HSO}}_3^ - {\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}}\)
Given that the \({K_{\text{a}}}\) for this equilibrium is \(1.25 \times {10^{ - 2}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), determine the pH of a \(2.00{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of sulfur dioxide.
Using Table 15 of the Data Booklet, identify an organic acid that is a stronger acid than sulfur dioxide.
Reaction kinetics can be investigated using the iodine clock reaction. The equations for two reactions that occur are given below.
Reaction A: \({{\text{H}}_2}{{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}\)
Reaction B: \({\text{ }}{{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} \to {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {{\text{S}}_4}{\text{O}}_6^{2 - }{\text{(aq)}}\)
Reaction B is much faster than reaction A, so the iodine, \({\text{I}_2}\), formed in reaction A immediately reacts with thiosulfate ions, \({{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\), in reaction B, before it can react with starch to form the familiar blue-black, starch-iodine complex.
In one experiment the reaction mixture contained:
5.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 2.00 \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrogen peroxide (\({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\))
5.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 1% aqueous starch
20.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 1.00 \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sulfuric acid (\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\))
20.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of 0.0100 \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium thiosulfate (\({\text{N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}\))
50.0 ± 0.1 \({\text{c}}{{\text{m}}^{\text{3}}}\) of water with 0.0200 ± 0.0001 g of potassium iodide (KI) dissolved in it.
After 45 seconds this mixture suddenly changed from colourless to blue-black.
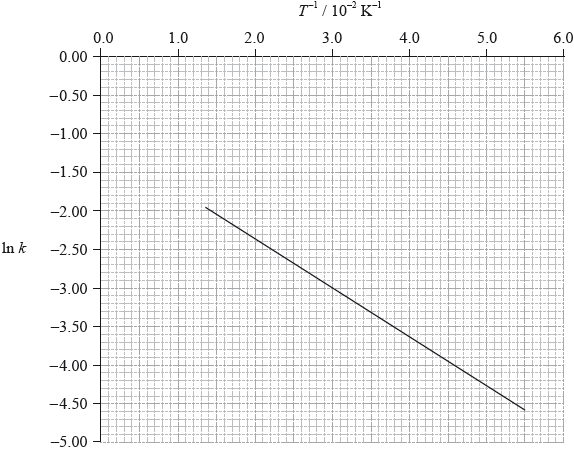
The activation energy can be determined using the Arrhenius equation, which is given in Table 1 of the Data Booklet. The experiment was carried out at five different temperatures. An incomplete graph to determine the activation energy of the reaction, based on these results, is shown below.
The concentration of iodide ions, \({{\text{I}}^ - }\), is assumed to be constant. Outline why this is a valid assumption.
For this mixture the concentration of hydrogen peroxide, \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\), can also be assumed to be constant. Explain why this is a valid assumption.
Explain why the solution suddenly changes colour.
Calculate the total uncertainty, in \({\text{c}}{{\text{m}}^{\text{3}}}\), of the volume of the reaction mixture.
Calculate the percentage uncertainty of the concentration of potassium iodide solution added to the overall reaction mixture.
Determine the percentage uncertainty in the concentration of potassium iodide in the final reaction solution.
The colour change occurs when \(1.00 \times {10^{ - 4}}{\text{ mol}}\) of iodine has been formed. Use the total volume of the solution and the time taken, to calculate the rate of the reaction, including appropriate units.
State the labels for each axis.
x-axis:
y-axis:
Use the graph to determine the activation energy of the reaction, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), correct to three significant figures.
In another experiment, 0.100 g of a black powder was also added while all other concentrations and volumes remained unchanged. The time taken for the solution to change colour was now 20 seconds. Outline why you think the colour change occurred more rapidly and how you could confirm your hypothesis.
Consider the following graph of \(\ln k\) against \(\frac{1}{T}\).
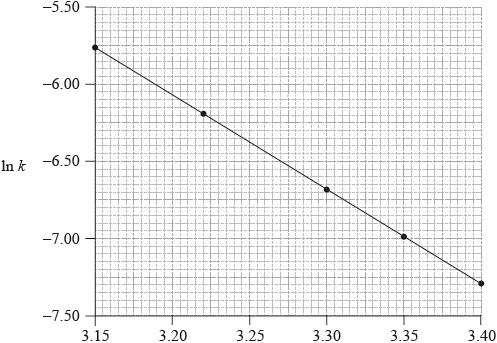
\[\frac{1}{T}/{10^{ - 3}}{\text{ }}{{\text{K}}^{ - 1}}\]
A catalyst provides an alternative pathway for a reaction, lowering the activation energy, \({E_{\text{a}}}\). Define the term activation energy, \({E_{\text{a}}}\).
State how the rate constant, k , varies with temperature, T.
Determine the activation energy, \({E_{\text{a}}}\), correct to three significant figures and state its units.
Alex and Hannah were asked to investigate the kinetics involved in the iodination of propanone. They were given the following equation by their teacher.
\[{\text{C}}{{\text{H}}_3}{\text{COC}}{{\text{H}}_3}{\text{(aq)}} + {{\text{I}}_2}{\text{(aq)}}\xrightarrow{{{{\text{H}}^ + }{\text{(aq)}}}}{\text{C}}{{\text{H}}_2}{\text{ICOC}}{{\text{H}}_3}{\text{(aq)}} + {\text{HI(aq)}}\]
Alex’s hypothesis was that the rate will be affected by changing the concentrations of the propanone and the iodine, as the reaction can happen without a catalyst. Hannah’s hypothesis was that as the catalyst is involved in the reaction, the concentrations of the propanone, iodine and the hydrogen ions will all affect the rate.
They carried out several experiments varying the concentration of one of the reactants or the catalyst whilst keeping other concentrations and conditions the same, and obtained the results below.
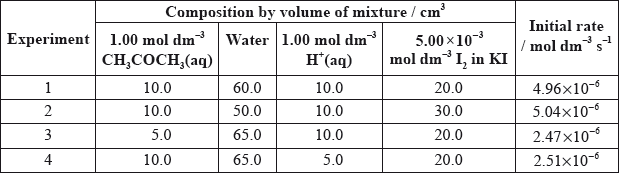
Explain why they added water to the mixtures.
(i) Deduce the order of reaction for each substance and the rate expression from the results.
(ii) Comment on whether Alex’s or Hannah’s hypothesis is correct.
Using the data from Experiment 1, determine the concentration of the substances used and the rate constant for the reaction including its units.
(i) This reaction uses a catalyst. Sketch and annotate the Maxwell-Boltzmann energy distribution curve for a reaction with and without a catalyst on labelled axes below.
(ii) Describe how a catalyst works.
Sodium thiosulfate solution, \({\text{N}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\), and hydrochloric acid, \({\text{HCl(aq)}}\), react to produce solid sulfur as in the equation below.
\[{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{S(s)}} + {\text{S}}{{\text{O}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}}\]
The following results to determine the initial rate were obtained:
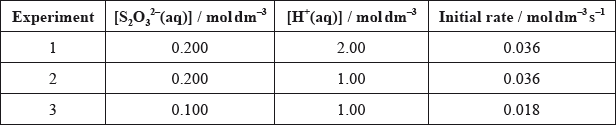
Deduce, with a reason, the order of reaction with respect to each reactant.
State the rate expression for this reaction.
Determine the value of the rate constant, \(k\), and state its units.
State an equation for a possible rate-determining step for the reaction.
Suggest how the activation energy, \({E_{\text{a}}}\), for this reaction may be determined.
The Haber process enables the large-scale production of ammonia needed to make fertilizers.
The equation for the Haber process is given below.
\[{{\text{N}}_2}({\text{g)}} + 3{{\text{H}}_2}({\text{g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_3}({\text{g)}}\]
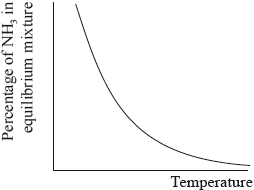
The percentage of ammonia in the equilibrium mixture varies with temperature.
Ammonia can be converted into nitric acid, \({\text{HN}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\), and hydrocyanic acid, HCN(aq). The \({\text{p}}{K_{\text{a}}}\) of hydrocyanic acid is 9.21.
A student decided to investigate the reactions of the two acids with separate samples of \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.
(i) Use the graph to deduce whether the forward reaction is exothermic or endothermic and explain your choice.
(ii) State and explain the effect of increasing the pressure on the yield of ammonia.
(iii) Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
(iv) A mixture of 1.00 mol \({{\text{N}}_{\text{2}}}\) and 3.00 mol \({{\text{H}}_{\text{2}}}\) was placed in a \({\text{1.0 d}}{{\text{m}}^{\text{3}}}\) flask at 400 °C. When the system was allowed to reach equilibrium, the concentration of was found to be \({\text{0.062 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Determine the equilibrium constant, \({K_{\text{c}}}\), of the reaction at this temperature.
(v) Iron is used as a catalyst in the Haber process. State the effect of a catalyst on the value of \({K_{\text{c}}}\).
(i) Distinguish between the terms strong and weak acid and state the equations used to show the dissociation of each acid in aqueous solution.
(ii) Deduce the expression for the ionization constant, \({K_{\text{a}}}\), of hydrocyanic acid and calculate its value from the \({\text{p}}{K_{\text{a}}}\) value given.
(iii) Use your answer from part (b) (ii) to calculate the \({\text{[}}{{\text{H}}^ + }{\text{]}}\) and the pH of an aqueous solution of hydrocyanic acid of concentration \({\text{0.108 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). State one assumption made in arriving at your answer.
A small piece of magnesium ribbon is added to solutions of nitric and hydrocyanic acid of the same concentration at the same temperature. Describe two observations that would allow you to distinguish between the two acids.
(i) Calculate the volume of the sodium hydroxide solution required to react exactly with a \({\text{15.0 c}}{{\text{m}}^{\text{3}}}\) solution of \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) nitric acid.
(ii) The following hypothesis was suggested by the student: “Since hydrocyanic acid is a weak acid it will react with a smaller volume of the \({\text{0.20 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution.” Comment on whether or not this is a valid hypothesis.
(iii) Use Table 16 of the Data Booklet to identify a suitable indicator for the titration of sodium hydroxide and hydrocyanic acid.
Chemical kinetics involves an understanding of how the molecular world changes with time.
A catalyst provides an alternative pathway for a reaction, lowering the activation energy, \({E_{\text{a}}}\).
Sketch graphical representations of the following reactions, for X \( \to \) products.
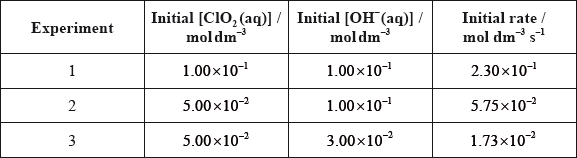
For the reaction below, consider the following experimental data.
\[{\text{2Cl}}{{\text{O}}_2}{\text{(aq)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq)}} \to {\text{ClO}}_3^ - {\text{(aq)}} + {\text{ClO}}_2^ - {\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}}\]
Another reaction involving \({\rm{O}}{{\rm{H}}^ - }\) (aq) is the base hydrolysis reaction of an ester.
\[{\text{C}}{{\text{H}}_3}{\text{COOC}}{{\text{H}}_2}{\text{CH(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}} \to {\text{C}}{{\text{H}}_3}{\text{CO}}{{\text{O}}^ - }{\text{(aq)}} + {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH(aq)}}\]
A two-step mechanism has been proposed for the following reaction.
\[\begin{array}{*{20}{l}} {{\text{Step 1:}}}&{{\text{Cl}}{{\text{O}}^ - }{\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}} \to {\text{ClO}}_2^ - {\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}} \\ {{\text{Step 2:}}}&{{\text{ClO}}_2^ - {\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}} \to {\text{ClO}}_3^ - {\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}} \end{array}\]
(i) Define the term rate of reaction.
(ii) Temperature and the addition of a catalyst are two factors that can affect the rate of a reaction. State two other factors.
(iii) In the reaction represented below, state one method that can be used to measure the rate of the reaction.
\[{\text{ClO}}_3^ - {\text{(aq)}} + {\text{5C}}{{\text{l}}^ - }{\text{(aq)}} + {\text{6}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{3C}}{{\text{l}}_2}{\text{(aq)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}\]
(i) Define the term activation energy, \({E_{\text{a}}}\).
(ii) Sketch the two Maxwell–Boltzmann energy distribution curves for a fixed amount of gas at two different temperatures, \({T_1}\) and \({T_2}{\text{ }}({T_2} > {T_1})\). Label both axes.

(i) Concentration of reactant X against time for a zero-order reaction.
(ii) Rate of reaction against concentration of reactant X for a zero-order reaction.
(iii) Rate of reaction against concentration of reactant X for a first-order reaction.
(i) Deduce the rate expression.
(ii) Determine the rate constant, \(k\), and state its units, using the data from Experiment 2.
(iii) Calculate the rate, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{{\text{s}}^{ - 1}}\), when \({\text{[Cl}}{{\text{O}}_2}{\text{(aq)]}} = 1.50 \times {10^{ - 2}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and \({\text{[O}}{{\text{H}}^ - }{\text{(aq)]}} = 2.35 \times {10^{ - 2}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
Apply IUPAC rules to name the ester, CH3COOCH2CH3(aq).
Describe qualitatively the relationship between the rate constant, k, and temperature, T.
The rate of this reaction was measured at different temperatures and the following data were recorded.
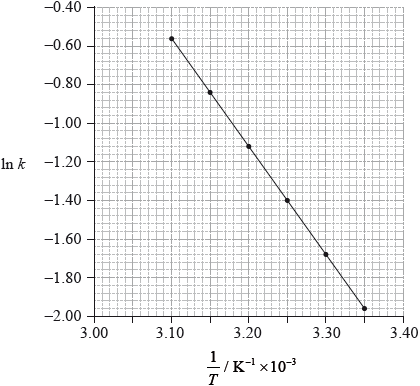
Using data from the graph, determine the activation energy, \({E_{\text{a}}}\), correct to three significant figures and state its units.
Deduce the overall equation for the reaction.
Deduce the rate expression for each step.
Step 1:
Step 2:
Hydrogen peroxide decomposes according to the equation below.
\({\text{2}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{(aq)}} \to {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} + {{\text{O}}_{\text{2}}}{\text{(g)}}\)
The rate of the decomposition can be monitored by measuring the volume of oxygen gas released. The graph shows the results obtained when a solution of hydrogen peroxide decomposed in the presence of a CuO catalyst.
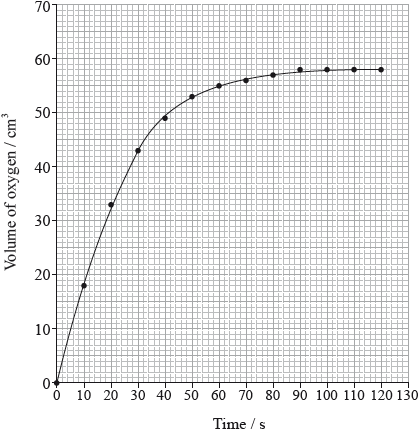
Outline how the initial rate of reaction can be found from the graph.
Explain how and why the rate of reaction changes with time.
A Maxwell-Boltzmann energy distribution curve is drawn below. Label both axes and explain, by annotating the graph, how catalysts increase the rate of reaction.
(i) In some reactions, increasing the concentration of a reactant does not increase the rate of reaction. Describe how this may occur.
(ii) Consider the reaction
\[{\text{2A}} + {\text{B}} \to {\text{C}} + {\text{D}}\]
The reaction is first order with respect to A, and zero order with respect to B. Deduce the rate expression for this reaction.
Sketch a graph of rate constant \((k)\) versus temperature.
Hydrochloric acid neutralizes sodium hydroxide, forming sodium chloride and water.
\({\text{NaOH(aq)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\) \(\Delta {H^\Theta } = - 57.9{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)
(i) Define standard enthalpy change of reaction, \(\Delta {H^\Theta }\).
(ii) Determine the amount of energy released, in kJ, when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydroxide solution reacts with \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) hydrochloric acid solution.
(iii) In an experiment, 2.50 g of solid sodium hydroxide was dissolved in \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of water. The temperature rose by 13.3 °C. Calculate the standard enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for dissolving one mole of solid sodium hydroxide in water.
\[{\text{NaOH(s)}} \to {\text{NaOH(aq)}}\]
(iv) Using relevant data from previous question parts, determine \(\Delta {H^\Theta }\), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), for the reaction of solid sodium hydroxide with hydrochloric acid.
\[{\text{NaOH(s)}} + {\text{HCl(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}}\]
(i) Zinc is found in the d-block of the periodic table. Explain why it is not considered a transition metal.
(ii) Explain why \({\text{F}}{{\text{e}}^{3 + }}\) is a more stable ion than \({\text{F}}{{\text{e}}^{2 + }}\) by reference to their electron configurations.
When nitrogen gas and hydrogen gas are allowed to react in a closed container the following equilibrium is established.
\[{{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2N}}{{\text{H}}_{\text{3}}}{\text{(g) }}\Delta H = -92.6{\text{ kJ}}\]
Outline two characteristics of a reversible reaction in a state of dynamic equilibrium.
Predict, with a reason, how each of the following changes affects the position of equilibrium.
The volume of the container is increased.
Ammonia is removed from the equilibrium mixture.
Define the term activation energy, \({E_{\text{a}}}\).
Ammonia is manufactured by the Haber process in which iron is used as a catalyst.
Explain the effect of a catalyst on the rate of reaction.
Typical conditions used in the Haber process are 500 °C and 200 atm, resulting in approximately 15% yield of ammonia.
(i) Explain why a temperature lower than 500 °C is not used.
(ii) Outline why a pressure higher than 200 atm is not often used.
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction on page 10.
When 1.00 mol of nitrogen and 3.00 mol of hydrogen were allowed to reach equilibrium in a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) container at a temperature of 500 °C and a pressure of 1000 atm, the equilibrium mixture contained 1.46 mol of ammonia.
Calculate the value of \({K_{\text{c}}}\) at 500 °C.
Define the term base according to the Lewis theory.
Define the term weak base according to the Brønsted–Lowry theory.
Deduce the formulas of conjugate acid-base pairs in the reaction below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}{\text{(aq)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{NH}}_{\text{3}}^ + {\text{(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\]
Determine the pH of a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}{\text{(aq)}}\), using tables 2 and 15 of the data booklet.
(i) Sketch the pH titration curve obtained when \({\text{50.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{N}}{{\text{H}}_{\text{3}}}{\text{(aq)}}\) is added to \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{HCl (aq)}}\).
(ii) Identify an indicator from table 16 of the data booklet that could be used for this titration.
Nitrogen(II) oxide reacts with hydrogen according to the equation below.
\[{\text{2NO(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{(g)}} \to {{\text{N}}_{\text{2}}}{\text{(g)}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(g)}}\]
A suggested mechanism for this reaction is:
Step 1: \({\text{NO}} + {{\text{H}}_{\text{2}}} \rightleftharpoons {\text{X}}\) fast
Step 2: \({\text{X}} + {\text{NO}} \to {\text{Y}} + {{\text{H}}_{\text{2}}}{\text{O}}\) slow
Step 3: \({\text{Y}} + {{\text{H}}_{\text{2}}} \to {{\text{N}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}}\) fast
Define the term rate of reaction.
Explain why increasing the particle size of a solid reactant decreases the rate of reaction.
Identify the rate-determining step.
A student hypothesized that the order of reaction with respect to \({{\text{H}}_{\text{2}}}\) is 2.
Evaluate this hypothesis.
A class studied the equilibrium established when ethanoic acid and ethanol react together in the presence of a strong acid, using propanone as an inert solvent. The equation is given below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}} + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
One group made the following initial mixture:
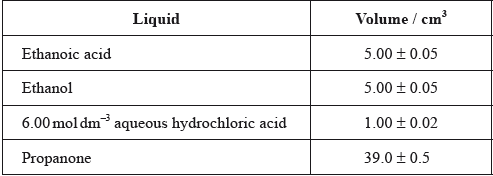
After one week, a \(5.00 \pm 0.05{\text{ c}}{{\text{m}}^{\text{3}}}\) sample of the final equilibrium mixture was pipetted out and titrated with \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide to determine the amount of ethanoic acid remaining. The following titration results were obtained:
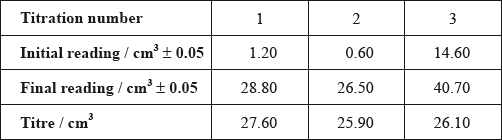
The density of ethanoic acid is \({\text{1.05 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\). Determine the amount, in mol, of ethanoic acid present in the initial mixture.
The concentration of ethanoic acid can be calculated as \({\text{1.748 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Determine the percentage uncertainty of this value. (Neglect any uncertainty in the density and the molar mass.)
Calculate the absolute uncertainty of the titre for Titration 1 (\({\text{27.60 c}}{{\text{m}}^3}\)).
Suggest the average volume of alkali, required to neutralize the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample, that the student should use.
\({\text{3.00 c}}{{\text{m}}^{\text{3}}}\) of the \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide reacted with the hydrochloric acid present in the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample. Determine the concentration of ethanoic acid in the final equilibrium mixture.
Deduce the equilibrium constant expression for the reaction.
The other concentrations in the equilibrium mixture were calculated as follows:
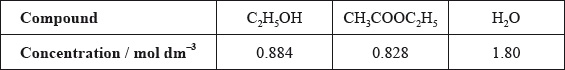
Use these data, along with your answer to part (iii), to determine the value of the equilibrium constant. (If you did not obtain an answer to part (iii), assume the concentrations of ethanol and ethanoic acid are equal, although this is not the case.)
Outline how you could establish that the system had reached equilibrium at the end of one week.
Outline why changing the temperature has only a very small effect on the value of the equilibrium constant for this equilibrium.
Outline how adding some ethyl ethanoate to the initial mixture would affect the amount of ethanoic acid converted to product.
Propanone is used as the solvent because one compound involved in the equilibrium is insoluble in water. Identify this compound and explain why it is insoluble in water.
Suggest one other reason why using water as a solvent would make the experiment less successful.
The rate of reaction is an important factor in industrial processes such as the Contact process to make sulfur trioxide, \({\text{S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\).
Define the term rate of reaction.
Describe the collision theory.
The Contact process involves this homogeneous equilibrium:
\[{\text{2S}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\,\,\,\,\,\Delta H = - 198{\text{ kJ}}\]
State and explain how increasing the pressure of the reaction mixture affects the yield of \({\text{S}}{{\text{O}}_{\text{3}}}\).
The Contact process involves this homogeneous equilibrium:
\[{\text{2S}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\,\,\,\,\,\Delta H = - 198{\text{ kJ}}\]
2.00 mol of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{(g)}}\) are mixed with 3.00 mol of \({{\text{O}}_{\text{2}}}{\text{(g)}}\) in a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) container until equilibrium is reached. At equilibrium there are 0.80 mol of \({\text{S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\).
Determine the equilibrium constant (\({K_{\text{c}}}\)) assuming all gases are at the same temperature and pressure.
The Contact process involves this homogeneous equilibrium:
\[{\text{2S}}{{\text{O}}_{\text{2}}}{\text{(g)}} + {{\text{O}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_{\text{3}}}{\text{(g)}}\,\,\,\,\,\Delta H = - 198{\text{ kJ}}\]
State the effect of increasing temperature on the value of \({K_{\text{c}}}\) for this reaction.
Outline the economic importance of using a catalyst in the Contact process.
The rate of the acid-catalysed iodination of propanone can be followed by measuring how the concentration of iodine changes with time.
I2(aq) + CH3COCH3(aq) → CH3COCH2I(aq) + H+(aq) + I−(aq)
The general form of the rate equation is:
Rate = [H3CCOCH3(aq)]m × [I2(aq)]n × [H+(aq)]p
The reaction is first order with respect to propanone.
Suggest how the change of iodine concentration could be followed.
A student produced these results with \([{{\text{H}}^ + }] = 0.15{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Propanone and acid were in excess and iodine was the limiting reagent. Determine the relative rate of reaction when \([{{\text{H}}^ + }] = 0.15{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\).
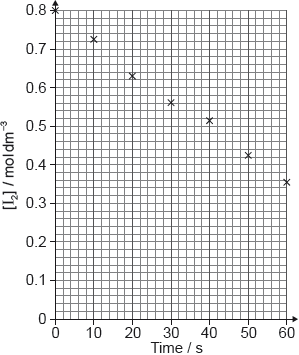
The student then carried out the experiment at other acid concentrations with all other conditions remaining unchanged.
Determine the relationship between the rate of reaction and the concentration of acid and the order of reaction with respect to hydrogen ions.
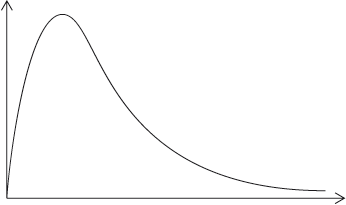
When the concentration of iodine is varied, while keeping the concentrations of acid and propanone constant, the following graphs are obtained.
Deduce, giving your reason, the order of reaction with respect to iodine.
When the reaction is carried out in the absence of acid the following graph is obtained.
Discuss the shape of the graph between A and B.
The reaction between hydrogen and nitrogen monoxide is thought to proceed by the mechanism shown below.
(i) State the equation for the overall reaction.
(ii) Deduce the rate expression consistent with this mechanism.
(iii) Explain how you would attempt to confirm this rate expression, giving the results you would expect.
(iv) State, giving your reason, whether confirmation of the rate expression would prove that the mechanism given is correct.
(v) Suggest how the rate of this reaction could be measured experimentally.
The enthalpy change for the reaction between nitrogen monoxide and hydrogen is −664 kJ and its activation energy is 63 kJ.
(i) Sketch the potential energy profile for the overall reaction, using the axes given, indicating both the enthalpy of reaction and activation energy.
(ii) This reaction is normally carried out using a catalyst. Draw a dotted line labelled “Catalysed” on the diagram above to indicate the effect of the catalyst.
(iii) Sketch and label a second Maxwell–Boltzmann energy distribution curve representing the same system but at a higher temperature, Thigher.
(iv) Explain why an increase in temperature increases the rate of this reaction.
One of the intermediates in the reaction between nitrogen monoxide and hydrogen is dinitrogen monoxide, N2O. This can be represented by the resonance structures below:
(i) Analyse the bonding in dinitrogen monoxide in terms of σ-bonds and Δ-bonds.
(ii) State what is meant by resonance.