
SL Paper 1
Lewis structures are represented in different ways in different parts of the world. Two ways of drawing the Lewis structure for \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) are shown below.
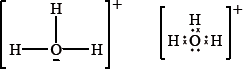
Which statement is correct about \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\)?
A. The ion has a tetrahedral shape.
B. The H–O–H bond angle is 120°.
C. The H–O–H bond angle is 90°.
D. The ion has a trigonal pyramidal shape.
Markscheme
D
Examiners report
One respondent stated that as the hydronium cation involves dative covalent bonding it would have been better if the dot-cross representation would have reflected this, which is a valid point. However, this did not stop candidates answering the question and 72.31% of candidates got the correct answer, namely that the ion has a trigonal pyramidal shape i.e. D.
Which compound has the lowest boiling point?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
Markscheme
D
Examiners report
Which is the best description of a metallic bond?
A. Electrostatic attraction between oppositely charged ions
B. Electrostatic attraction between a pair of electrons and positively charged nuclei
C. Electrostatic attraction between a lattice of positive ions and delocalized electrons
D. Electrostatic attraction for a bonding pair of electrons which have been supplied by one of the atoms
Markscheme
C
Examiners report
Which statements are correct about hydrogen bonding?
I. It is an electrostatic attraction between molecules.
II. It is present in liquid ammonia.
III. It is a permanent dipole-permanent dipole attraction.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
What describes the structure of silicon and silicon dioxide?
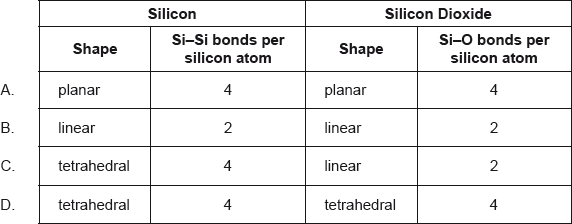
Markscheme
D
Examiners report
This question was straight from the Assessment Statement 4.2.10 and some thought it was a tough but fair question. Only 22.10% answered it correctly. Both silicon and silicon dioxide have giant covalent structures, but the most common answers were B and C suggesting that students think these are linear molecules.
Which diagram represents the bonding in \({\text{Si}}{{\text{O}}_{\text{2}}}\)?
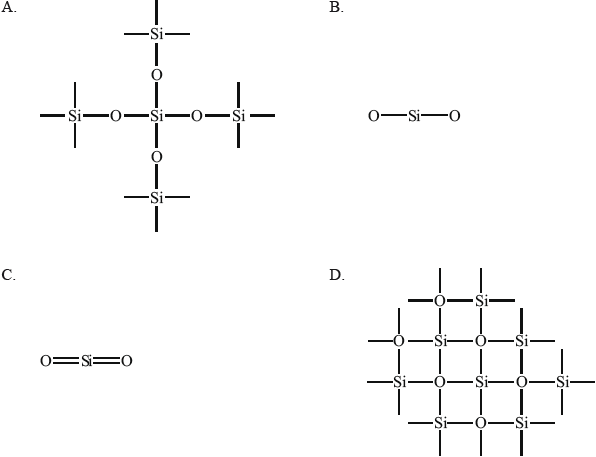
Markscheme
A
Examiners report
This question was written in response to poor paper 2 answers in November 2011. The two-dimensional representation of the bonding was chosen to make the question easier for candidates. The question is about bonding and not structure and was designed to test one thing specifically; over 43% of candidates thought silicon dioxide to have the same structure as carbon dioxide, answer C.
Which change explains why the boiling points of the halogens increase as their molecular masses increase?
A. The intermolecular attraction due to temporarily induced dipoles increases.
B. The gravitational attraction between molecules increases.
C. The polarity of the bond within the molecule increases.
D. The strength of the bond within the molecule increases.
Markscheme
A
Examiners report
The formula of gallium phosphate is \({\text{GaP}}{{\text{O}}_{\text{4}}}\). What is the correct formula of gallium sulfate?
A. \({\text{GaS}}{{\text{O}}_{\text{4}}}\)
B. GaS
C. \({\text{G}}{{\text{a}}_{\text{2}}}{{\text{(S}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{3}}}\)
D. \({\text{G}}{{\text{a}}_{\text{2}}}{{\text{S}}_{\text{3}}}\)
Markscheme
C
Examiners report
Which compound has a covalent macromolecular (giant covalent) structure?
A. MgO(s)
B. \({\text{A}}{{\text{l}}_{\text{2}}}{{\text{O}}_{\text{3}}}{\text{(s)}}\)
C. \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}{\text{(s)}}\)
D. \({\text{Si}}{{\text{O}}_{\text{2}}}{\text{(s)}}\)
Markscheme
D
Examiners report
One respondent stated that the terminology covalent macromolecular was unfamiliar. All candidates should be familiar with covalent and an alternative to macromolecular is giant which was given in brackets in the question. The question itself was answered correctly by 60% of candidates.
Which metal has the strongest metallic bond?
A. Li
B. Na
C. K
D. Rb
Markscheme
A
Examiners report
Which substance is made up of a lattice of positive ions and free moving electrons?
A. Graphite
B. Sodium chloride
C. Sulfur
D. Sodium
Markscheme
D
Examiners report
A substance has the following properties:
What is the most probable structure of this substance?
A. Network covalent
B. Polar covalent molecule
C. Ionic lattice
D. Metallic lattice
Markscheme
A
Examiners report
Which species contain a dative covalent (coordination or coordinate) bond?
I. Carbon monoxide, CO
II. Ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}\)
III. Oxonium ion, \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
One respondent would have preferred “hydronium” ion although the ion was made clear by the formula. Over half the candidates gave the correct answer.
Which molecule is non-polar?
A. \({\text{CC}}{{\text{l}}_{\text{4}}}\)
B. \({\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}}\)
D. CO
Markscheme
A
Examiners report
The most common misconception was that CO is non-polar.
What are the correct formulas of the following ions?
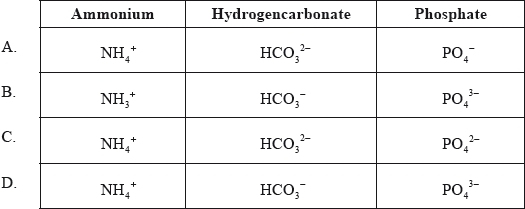
Markscheme
D
Examiners report
Which statement about the physical properties of substances is correct?
A. The only solids that conduct electricity are metals.
B. All substances with covalent bonds have low melting points.
C. Ionic solids are always brittle.
D. All metals have high densities.
Markscheme
C
Examiners report
Which molecule has a non-bonding (lone) pair of electrons on the central atom?
A. \({\text{B}}{{\text{F}}_{\text{3}}}\)
B. \({\text{S}}{{\text{O}}_{\text{2}}}\)
C. \({\text{C}}{{\text{O}}_{\text{2}}}\)
D. \({\text{Si}}{{\text{F}}_{\text{4}}}\)
Markscheme
B
Examiners report
What is the formula of magnesium fluoride?
A. \({\text{M}}{{\text{g}}_{\text{2}}}{{\text{F}}_{\text{3}}}\)
B. \({\text{M}}{{\text{g}}_{\text{2}}}{\text{F}}\)
C. \({\text{M}}{{\text{g}}_{\text{3}}}{{\text{F}}_{\text{2}}}\)
D. \({\text{Mg}}{{\text{F}}_{\text{2}}}\)
Markscheme
D
Examiners report
Which statements about the structure and bonding of silicon dioxide are correct?
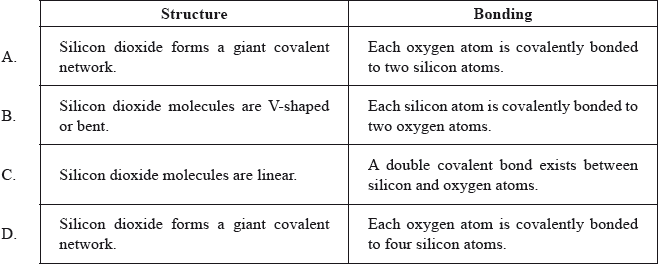
Markscheme
A
Examiners report
Which particles are responsible for electrical conductivity in metals?
A. Anions
B. Cations
C. Electrons
D. Protons
Markscheme
C
Examiners report
One G2 comment stated that the terms cation and anion are not stated on the syllabus. Although strictly correct, it would be assumed that these terms would be introduced to students in the classroom as they are universally used in chemistry (e.g. even the term carbocation is widely used in explaining certain nucleophilic substitution reaction mechanisms).
What are the correct formulas of the following ions?
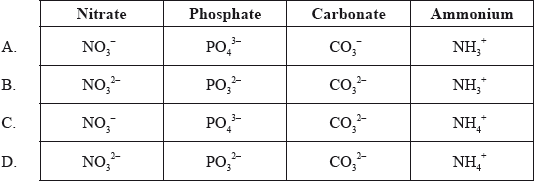
Markscheme
C
Examiners report
What is the correct Lewis structure for hypochlorous acid, a compound containing chlorine, hydrogen and oxygen?
A. 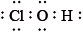
B. 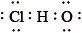
C. 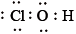
D. 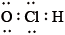
Markscheme
C
Examiners report
What is the correct order of increasing boiling points?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Cl}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{I}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Cl}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{I}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{I}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Cl}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Cl}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{I}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
Markscheme
A
Examiners report
What is the order of increasing boiling point?
A. C4H10 < CH3COOH < CH3CH2CHO < CH3CH2CH2OH
B. C4H10 < CH3CH2CHO < CH3CH2CH2OH < CH3COOH
C. CH3COOH < CH3CH2CH2OH< CH3CH2CHO < C4H10
D. C4H10 < CH3CH2CH2OH < CH3CH2CHO < CH3COOH
Markscheme
B
Examiners report
Metal M has only one oxidation number and forms a compound with the formula \({\text{MC}}{{\text{O}}_{\text{3}}}\). Which formula is correct?
A. \({\text{MN}}{{\text{O}}_{\text{3}}}\)
B. \({\text{MN}}{{\text{H}}_{\text{4}}}\)
C. \({\text{MS}}{{\text{O}}_{\text{4}}}\)
D. \({\text{MP}}{{\text{O}}_{\text{4}}}\)
Markscheme
C
Examiners report
The question proved surprisingly challenging, as indicated by a high number of blank responses and a difficulty index of 55%. This would seem to indicate that a disturbing number of candidates are not aware of the charges on the common ions. It was however a good discriminator with a discrimination index of 0.55.
Which is the correct Lewis structure for ethene?
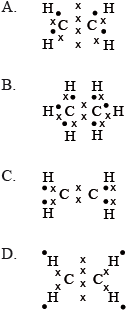
Markscheme
A
Examiners report
There were four G2 comments on this question all of which stated that some of the Lewis structures for ethane were not represented clear enough, particularly in relation to choice C, which is a valid comment and this will be taken on board in future paper settings. In the case of choice A one respondent stated that it would have been better to represent the carbon to carbon double bond in the Lewis structure as C:::C instead of having the electrons shown in a vertical line. However, candidates should realise that electrons in Lewis structural representations can be represented in a variety of ways and hence teachers should ensure that students in class get ample practice of writing Lewis structures in different ways.
Which order is correct when the following compounds are arranged in order of increasing melting point?
A. \({\text{C}}{{\text{H}}_{\text{4}}} < {{\text{H}}_{\text{2}}}{\text{S}} < {{\text{H}}_{\text{2}}}{\text{O}}\)
B. \({{\text{H}}_{\text{2}}}{\text{S}} < {{\text{H}}_{\text{2}}}{\text{O}} < {\text{C}}{{\text{H}}_{\text{4}}}\)
C. \({\text{C}}{{\text{H}}_{\text{4}}} < {{\text{H}}_{\text{2}}}{\text{O}} < {{\text{H}}_{\text{2}}}{\text{S}}\)
D. \({{\text{H}}_{\text{2}}}{\text{S}} < {\text{C}}{{\text{H}}_{\text{4}}} < {{\text{H}}_{\text{2}}}{\text{O}}\)
Markscheme
A
Examiners report
One respondent stated that it would be best to write from least reactive to most reactive in both of these questions. However, “increasing” is written in bold in both questions and, also, this type of question has been asked extensively on previous papers and hence candidates would have understood what was asked for explicitly if they had looked at some of the previous examination papers. In the case of Q.13 60% of candidates gave the correct answer and in Q.27, 68% had the question correct.
Which molecules react to form a dative covalent (coordinate) bond?
A. \({\text{C}}{{\text{H}}_{\text{4}}}\) and \({\text{N}}{{\text{H}}_{\text{3}}}\)
B. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\) and \({\text{C}}{{\text{l}}_{\text{2}}}\)
C. \({\text{N}}{{\text{H}}_{\text{3}}}\) and HF
D. Cl2 and HF
Markscheme
C
Examiners report
Students found this question on dative (coordinate) covalent bond to be difficult with 35.13% correct answers. However, choices A and B were commonly selected. In A, the carbon octet is full and cannot bond with the lone electron pair on \({\text{N}}{{\text{H}}_{\text{3}}}\); in B, the reaction of \({\text{C}}{{\text{l}}_{\text{2}}}\) with \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\) would be an addition reaction with covalent bond between C and Cl.
Which combination of length and strength of the carbon‒to‒carbon bonds in \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\) and \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\) is correct?
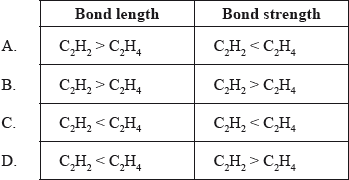
Markscheme
D
Examiners report
Which compound does not form hydrogen bonds between its molecules?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
Markscheme
B
Examiners report
What is the formula of magnesium nitride?
A. MgN
B. Mg2N3
C. Mg3N
D. Mg3N2
Markscheme
D
Examiners report
Which series shows increasing boiling points?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
Markscheme
D
Examiners report
The Lewis (electron dot) structure of paracetamol (acetaminophen) is:
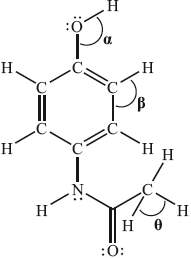
What are the approximate values of the bond angles?
Markscheme
A
Examiners report
In one G2 comment it was stated that since the question is about bond angles then it would be better to use a 3D representation of paracetamol. This was not the intention of the question. Candidates had to look at the number of negative charge centres (electron domains) around the two carbon atoms and the oxygen atom in order to relate this to the associated bond angle. In the case of the oxygen atom, there are four negative charge centres suggesting that the electron domain geometry is tetrahedral but the molecular geometry is actually v-shaped (bent). Due to the lone-pair/lone-pair repulsion, the actual bond angle is reduced from the ideal bond angle of 109.5° for \(\alpha \). For the two carbon atoms, one has three negative charge centres, implying a 120° bond angle and the other has four negative charge centres suggesting a 109.5° bond angle based on a tetrahedral molecular geometry around the carbon. 63.16% of candidates got the correct answer A. The question also had a reasonably good discrimination index of 0.55. Many candidates opted for D and simply took the bond angle based on the Lewis structure to be 90° for the H–C–H bond. This shows again the importance of introducing the 3D nature of molecules in the teaching of geometry as part of the teaching programme. Candidates should be exposed to constructing simple 3D molecules in class (and/or engaging with computer-aided visualizations if facilities allow) and candidates should understand the inherent differences between Lewis (electron dot) structures (which do not necessarily convey angular perspectives) and ball and stick type or other similar 3D representations. VSEPR theory should be employed as a useful model in bridging these two types of representations and this is especially important in looking at structures in the teaching of organic chemistry, where 2D structural formulas are often used.
Which compounds have an ionic lattice structure in the solid state?
I. Silicon dioxide
II. Sodium fluoride
III. Ammonium nitrate
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
Which forces are present between molecules of carbon dioxide in the solid state?
A. Permanent dipole-permanent dipole interactions
B. Temporary dipole-induced dipole interactions (London/dispersion forces)
C. Covalent bonding
D. Ionic bonding
Markscheme
B
Examiners report
Diamond, C60 fullerene and graphite are allotropes of carbon. Which statements are correct about these allotropes?
I. In diamond each carbon is held in a tetrahedral arrangement.
II. In C60 fullerene each carbon is held in a trigonal arrangement.
III. In graphite each carbon is held in a tetrahedral arrangement.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
A
Examiners report
Which compound has the shortest C–N bond?
A. CH3NH2
B. (CH3)3CNH2
C. CH3CN
D. CH3CHNH
Markscheme
C
Examiners report
Which combination of shape and bond angle best describes a molecule of sulfur dioxide, \({\text{S}}{{\text{O}}_{\text{2}}}\)?
Markscheme
C
Examiners report
What are the correct formulas of the following ions?
Markscheme
A
Examiners report
Which statement best describes ionic bonding?
A. It is the electrostatic attraction between positive ions and delocalized electrons and occurs by the transfer of electrons.
B. It is the electrostatic attraction between positive ions and negative ions and occurs by the transfer of electrons.
C. It is the electrostatic attraction between positive ions and negative ions and occurs by the sharing of electrons.
D. It is the electrostatic attraction between positive nuclei and electrons and occurs by the sharing of electrons.
Markscheme
B
Examiners report
How many bonding electrons are there in the urea molecule?
A. 8
B. 16
C. 20
D. 24
Markscheme
B
Examiners report
Which molecule contains a bond angle of approximately 120°?
A. \({\text{C}}{{\text{H}}_{\text{4}}}\)
B. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\)
C. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
D. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\)
Markscheme
C
Examiners report
Which two atoms form the most polar bond?
A. C and F
B. C and Cl
C. Si and F
D. Si and Cl
Markscheme
C
Examiners report
Between which pair of molecules can hydrogen bonding occur?
A. CH4 and H2O
B. CH3OCH3 and CF4
C. CH4 and HF
D. CH3OH and H2O
Markscheme
D
Examiners report
Which molecule has the shortest bond between carbon atoms?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\)
B. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
C. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\)
D. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}\)
Markscheme
C
Examiners report
What is the formula of calcium nitride?
A. \({\text{C}}{{\text{a}}_{\text{3}}}{{\text{N}}_{\text{2}}}\)
B. \({\text{C}}{{\text{a}}_{\text{2}}}{{\text{N}}_{\text{3}}}\)
C. \({\text{Ca(N}}{{\text{O}}_{\text{2}}}{{\text{)}}_{\text{2}}}\)
D. \({\text{Ca(N}}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\)
Markscheme
A
Examiners report
Which pair of molecules has the same bond angles?
A. PCl3 and BCl3
B. SO2 and CO2
C. H2O and NH3
D. CCl4 and SiH4
Markscheme
D
Examiners report
What are the approximate bond angles and structure of crystalline SiO2?
Markscheme
B
Examiners report
Which form of carbon is the poorest electrical conductor?
A. Graphite
B. Graphene
C. Diamond
D. Carbon nanotube
Markscheme
C
Examiners report
Which substance does not conduct electricity?
A. Solid zinc
B. Molten zinc
C. Solid zinc chloride
D. Molten zinc chloride
Markscheme
C
Examiners report
Which combination best describes the type of bonding present and the melting point of silicon and silicon dioxide?
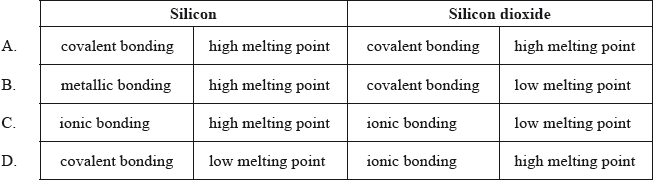
Markscheme
A
Examiners report
What is the formula of the ionic compound formed when calcium and nitrogen react together?
A. \({\text{C}}{{\text{a}}_{\text{2}}}{{\text{N}}_{\text{3}}}\)
B. \({\text{C}}{{\text{a}}_{\text{3}}}{{\text{N}}_{\text{2}}}\)
C. \({\text{C}}{{\text{a}}_{\text{5}}}{{\text{N}}_{\text{2}}}\)
D. \({\text{C}}{{\text{a}}_{\text{2}}}{{\text{N}}_{\text{5}}}\)
Markscheme
B
Examiners report
Which statements concerning the sodium chloride ionic lattice are correct?
I. Sodium ions are larger than chloride ions.
II. Each sodium ion is surrounded by six chloride ions.
III. Each chloride ion is surrounded by six sodium ions.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
What is the molecular geometry and bond angle in the molecular ion NO3−?
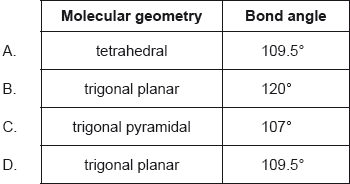
Markscheme
B
Examiners report
Which statements about graphite are correct?
I. Carbon atoms are held in layers with weak attractions between layers.
II. Graphite is a non-metal which conducts electricity.
III. Each carbon atom is covalently bonded to three other carbon atoms.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
D
Examiners report
What describes the relationship between diamond, graphite and \({{\text{C}}_{{\text{60}}}}\) fullerene?
A. Allotropes
B. Isomers
C. Isotopes
D. Polymers
Markscheme
A
Examiners report
What is the correct order of increasing boiling point?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}} < {\text{HCHO}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\)
B. \({\text{HCHO}} < {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}} < {\text{HCHO}} < {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\)
D. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}} < {\text{HCHO}}\)
Markscheme
A
Examiners report
How do the bond angles in \({\text{C}}{{\text{H}}_{\text{4}}}\), \({\text{N}}{{\text{H}}_{\text{3}}}\) and \({{\text{H}}_{\text{2}}}{\text{O}}\) compare?
\(\begin{array}{*{20}{l}} {{\text{A.}}}&{{\text{C}}{{\text{H}}_{\text{4}}}}& = &{{\text{N}}{{\text{H}}_{\text{3}}}}& = &{{{\text{H}}_{\text{2}}}{\text{O}}} \\ {{\text{B.}}}&{{\text{C}}{{\text{H}}_{\text{4}}}}& < &{{\text{N}}{{\text{H}}_{\text{3}}}}& < &{{{\text{H}}_{\text{2}}}{\text{O}}} \\ {{\text{C.}}}&{{\text{N}}{{\text{H}}_{\text{3}}}}& < &{{\text{C}}{{\text{H}}_{\text{4}}}}& < &{{{\text{H}}_{\text{2}}}{\text{O}}} \\ {{\text{D.}}}&{{{\text{H}}_{\text{2}}}{\text{O}}}& < &{{\text{N}}{{\text{H}}_{\text{3}}}}& < &{{\text{C}}{{\text{H}}_{\text{4}}}} \end{array}\)
Markscheme
D
Examiners report
What compound is formed when lithium reacts with selenium?
A. LiSe
B. \({\text{L}}{{\text{i}}_{\text{2}}}{\text{Se}}\)
C. \({\text{LiS}}{{\text{e}}_{\text{2}}}\)
D. \({\text{L}}{{\text{i}}_{\text{2}}}{\text{S}}{{\text{e}}_{\text{2}}}\)
Markscheme
B
Examiners report
Which properties do typical ionic compounds have?
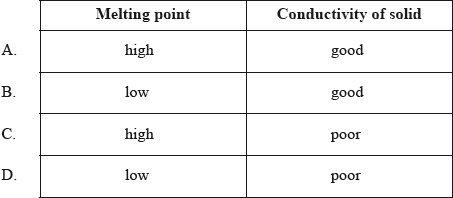
Markscheme
C
Examiners report
What is the formula of magnesium nitride?
A. \({\text{M}}{{\text{g}}_{\text{2}}}{{\text{N}}_{\text{3}}}\)
B. \({\text{M}}{{\text{g}}_{\text{3}}}{{\text{N}}_{\text{2}}}\)
C. \({\text{Mg(N}}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\)
D. \({\text{Mg(N}}{{\text{O}}_{\text{2}}}{{\text{)}}_{\text{2}}}\)
Markscheme
B
Examiners report
Which substance can form intermolecular hydrogen bonds in the liquid state?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OC}}{{\text{H}}_{\text{3}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
Markscheme
B
Examiners report
Which particles are responsible for the conduction of electricity in molten aluminium?
A. Cations
B. Anions
C. Electrons
D. Protons
Markscheme
C
Examiners report
There were a number of comments on this question and many teachers stated that although they assumed that the required answer was C. i.e. electrons, many felt that as molten aluminium was involved, the cations are mobile and thus could conduct electricity, so A. could be another answer. Although the correct answer C. (electrons) was given by the majority of candidates (71.18%), it was decided at Grade Award to also accept A. as clearly some candidates may have approached the question in the sense articulated by several teachers.
What are the predicted electron domain geometries around the carbon and both nitrogen atoms in urea, (NH2)2CO, applying VSEPR theory?
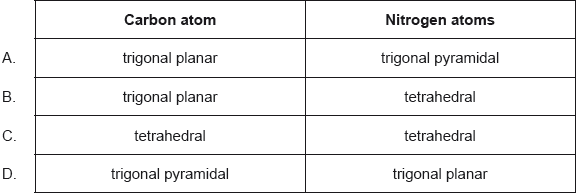
Markscheme
B
Examiners report
Which compound has the highest boiling point?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
Markscheme
C
Examiners report
The Lewis (electron dot) structure of aspirin is represented below.
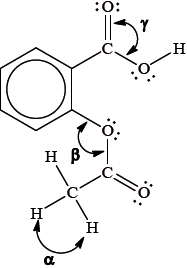
What are the approximate values of the bond angles \(\alpha \), \(\beta \) and \(\gamma \), in the molecule?
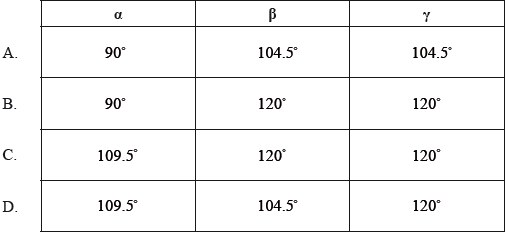
Markscheme
D
Examiners report
Which particles are present in the lattice of a metal?
A. Negative ions
B. Positive and negative ions
C. Positive ions
D. Molecules
Markscheme
Examiners report
C
Which statement best describes metallic bonding?
A. Electrostatic attractions between oppositely charged ions
B. Electrostatic attractions between a lattice of positive ions and delocalized electrons
C. Electrostatic attractions between a lattice of negative ions and delocalized protons
D. Electrostatic attractions between protons and electrons
Markscheme
B
Examiners report
The number of electrons in the valence shell of elements A and B, are 6 and 7 respectively. What is the formula and type of bonding in a compound formed by these elements?
A. \({{\text{A}}_{\text{2}}}{\text{B}}\), covalent
B. \({\text{A}}{{\text{B}}_{\text{2}}}\), covalent
C. \({{\text{A}}_{\text{2}}}{\text{B}}\), ionic
D. \({\text{A}}{{\text{B}}_{\text{2}}}\), ionic
Markscheme
B
Examiners report
Which statement best describes the intramolecular bonding in HCN(l)?
A. Electrostatic attractions between \({{\text{H}}^ + }\) and \({\text{C}}{{\text{N}}^ - }\) ions
B. Only van der Waals’ forces
C. Van der Waals’ forces and hydrogen bonding
D. Electrostatic attractions between pairs of electrons and positively charged nuclei
Markscheme
D
Examiners report
This was by far the most challenging question on the paper, with a difficulty index of 24%, and many teachers commented about it on the G2 form. It appears that at SL many candidates were not familiar with the term “intramolecular” and in addition failed to assume that the pure liquid compound was being referred to, both of which seemed to create a degree of confusion. It did however appear more accessible to the better candidates, with a discrimination index of 0.18.
Which statements are correct for the bonds between two carbon atoms?
I. Single bonds are longer than triple bonds.
II. Single bonds are stronger than double bonds.
III. Triple bonds are stronger than double bonds.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
What is the shape of the ammonia molecule, \({\text{N}}{{\text{H}}_{\text{3}}}\)?
A. Trigonal planar
B. Trigonal pyramidal
C. Linear
D. V-shaped (bent)
Markscheme
B
Examiners report
Which molecule is non-polar?
A. OF2
B. NH3
C. BF3
D. SO2
Markscheme
C
Examiners report
\({{\text{C}}_{{\text{60}}}}\) fullerene consists of a simple molecular structure. Silicon dioxide, \({\text{Si}}{{\text{O}}_{\text{2}}}\), can be described as a giant covalent (macromolecular) structure. Which statements are correct?
I. Each carbon atom in \({{\text{C}}_{{\text{60}}}}\) fullerene is bonded in a sphere of 60 carbon atoms, consisting of pentagons and hexagons.
II. Each O–Si–O bond angle in \({\text{Si}}{{\text{O}}_{\text{2}}}\) is 180°.
III. \({\text{Si}}{{\text{O}}_{\text{2}}}\) is insoluble in water.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
One respondent stated that statement I. could have been better worded and stated that bonded in a sphere could be taken to mean that each atom is bonded in a sphere rather than a sphere made of all 60 atoms. This is a fair comment. 41.13% of candidates got the correct answer B and the question was the sixth hardest question on the paper.
Which pair has the same bond angles?
A. \({\text{C}}{{\text{H}}_{\text{4}}}{\text{ and NH}}_{\text{4}}^ + \)
B. \({\text{N}}{{\text{H}}_{\text{3}}}{\text{ and }}{{\text{H}}_{\text{2}}}{\text{O}}\)
C. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{ and }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\)
D. \({\text{C}}{{\text{O}}_{\text{2}}}{\text{ and S}}{{\text{O}}_{\text{2}}}\)
Markscheme
A
Examiners report
Which process involves the breaking of hydrogen bonds?
A. \({\text{2HI(g)}} \to {{\text{H}}_{\text{2}}}{\text{(g)}} + {{\text{I}}_{\text{2}}}{\text{(g)}}\)
B. \({\text{C}}{{\text{H}}_{\text{4}}}{\text{(g)}} \to {\text{C(g)}} + {\text{4H(g)}}\)
C. \({{\text{H}}_{\text{2}}}{\text{(l)}} \to {{\text{H}}_{\text{2}}}{\text{(g)}}\)
D. \({\text{N}}{{\text{H}}_{\text{3}}}{\text{(l)}} \to {\text{N}}{{\text{H}}_{\text{3}}}{\text{(g)}}\)
Markscheme
D
Examiners report
Many considered B, the breaking of C–H bonds, to be correct.
When \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\), \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\) and \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\) are arranged in order of increasing carbon-carbon bond strength (weakest bond first), what is the correct order?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{, }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{, }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\)
B. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{, }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{, }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
C. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{, }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{, }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\)
D. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}{\text{, }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{, }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
Markscheme
C
Examiners report
What is the shape and the bond angle of the molecule \({\text{B}}{{\text{F}}_{\text{3}}}\)?
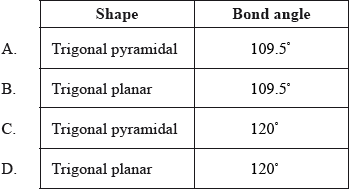
Markscheme
D
Examiners report
Which species contains a bond angle of approximately 107°?
A. \({{\text{H}}_{\text{2}}}{\text{O}}\)
B. \({\text{C}}{{\text{F}}_{\text{4}}}\)
C. \({\text{NC}}{{\text{l}}_{\text{3}}}\)
D. \({\text{B}}{{\text{F}}_{\text{3}}}\)
Markscheme
C
Examiners report
Which bond is the least polar?
A. C–H
B. F–H
C. O–H
D. N–H
Markscheme
A
Examiners report
What is the formula of calcium phosphide?
A. \({\text{C}}{{\text{a}}_{\text{2}}}{{\text{(P}}{{\text{O}}_{\text{3}}}{\text{)}}_{\text{3}}}\)
B. \({\text{C}}{{\text{a}}_{\text{2}}}{{\text{P}}_{\text{3}}}\)
C. \({\text{C}}{{\text{a}}_{\text{3}}}{{\text{(P}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{2}}}\)
D. \({\text{C}}{{\text{a}}_{\text{3}}}{{\text{P}}_{\text{2}}}\)
Markscheme
D
Examiners report
Which of the following are van der Waals’ forces?
I. Dipole-dipole forces
II. Hydrogen bonds
III. London (dispersion) forces
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
Which is the best description of ionic bonding?
A. The electrostatic attraction between positively charged nuclei and an electron pair
B. The electrostatic attraction between positive ions and delocalized negative ions
C. The electrostatic attraction between positive ions and delocalized electrons
D. The electrostatic attraction between oppositely charged ions
Markscheme
D
Examiners report
Which single covalent bond is the most polar, given the following electronegativity values?
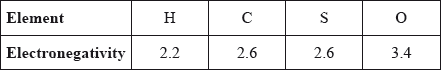
A. C–O
B. S–H
C. C–H
D. O–H
Markscheme
D
Examiners report
Which bonds are arranged in order of increasing polarity?
A. H–F \( < \) H–Cl \( < \) H–Br \( < \) H–I
B. H–I \( < \) H–Br \( < \) H–F \( < \) H–Cl
C. H–I \( < \) H–Br \( < \) H–Cl \( < \) H–F
D. H–Br \( < \) H–I \( < \) H–Cl \( < \) H–F
Markscheme
C
Examiners report
How many non-bonding pairs of electrons are there in a nitrogen molecule?
A. 0
B. 1
C. 2
D. 3
Markscheme
C
Examiners report
Which compound forms hydrogen bonds in the liquid state?
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}\)
B. \({\text{CHC}}{{\text{l}}_{\text{3}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\)
D. \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{3}}}{\text{N}}\)
Markscheme
A
Examiners report
One respondent stated that there are two correct answers to this question, namely A. and C. This is incorrect as C. is \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\) which is an aldehyde, and this does not form hydrogen bonding between its molecules. Hence the only correct answer is A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}\).
The compounds shown below have similar relative molecular masses. What is the correct order of increasing boiling point?
A. CH3COOH < (CH3)2CO < (CH3)2CHOH
B. CH3COOH < (CH3)2CHOH < (CH3)2CO
C. (CH3)2CO < CH3COOH < (CH3)2CHOH
D. (CH3)2CO < (CH3)2CHOH < CH3COOH
Markscheme
D
Examiners report
Which compound contains both ionic and covalent bonds?
A. SIH4
B. NaNO3
C. H2CO
D. Na2S
Markscheme
B
Examiners report
Which of the following series shows increasing hydrogen bonding with water?
A. Propane < propanal < propanol < propanoic acid
B. Propane < propanol < propanal < propanoic acid
C. Propanal < propane < propanoic acid < propanol
D. Propanoic acid < propanol < propanal < propane
Markscheme
A
Examiners report
Which combination of the characteristics of element X, a metal, and element Y, a non metal, is most likely to lead to ionic bonding?
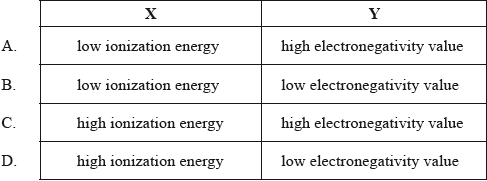
Markscheme
A
Examiners report
Which species contains a dative covalent (coordinate) bond?
A. HCN
B. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\)
C. \({\text{C}}{{\text{O}}_{\text{2}}}\)
D. CO
Markscheme
D
Examiners report
Many thought that HCN would contain a dative bond.
The following compounds have similar molar masses:
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH, C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH and C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\]
What is the order of increasing boiling points?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\)
Markscheme
D
Examiners report
The electronegativity values of four elements are given.
What is the order of increasing polarity of the bonds in the following compounds?
A. CO < OF2 < NO < CF4
B. CF4 < CO < OF2 < NO
C. NO < OF2 < CO < CF4
D. CF4 < NO < OF2 < CO
Markscheme
C
Examiners report
Which bonds cause the boiling point of water to be significantly greater than that of hydrogen sulfide?
A. London (dispersion)
B. Covalent
C. Ionic
D. Hydrogen
Markscheme
D
Examiners report
Which diatomic molecule has the strongest bonding between its atoms?
A. \({{\text{H}}_{\text{2}}}\)
B. \({{\text{N}}_{\text{2}}}\)
C. \({{\text{O}}_{\text{2}}}\)
D. \({{\text{F}}_{\text{2}}}\)
Markscheme
B
Examiners report
This caused some difficulties for candidates with opinion evenly divided between B (\({{\text{N}}_{\text{2}}}\), correct) and D (\({{\text{F}}_{\text{2}}}\)). Candidates were presumably thinking about electronegativity rather than the number of bonds between the atoms.
Which compounds contain both ionic and covalent bonding?
I. \({\text{CaC}}{{\text{O}}_{\text{3}}}\)
II. NaCl
III. NaOH
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
This was thought to be a fair question despite one respondent’s worry that there would be ambiguity for candidates who believe NaCl has a small degree of covalency. 78% gave the expected answer.
What is the formula of ammonium phosphate?
A. (NH3)3PO4
B. (NH4)3PO4
C. (NH4)2PO4
D. (NH3)2PO3
Markscheme
B
Examiners report
Which is the best description of the bonding present in silicon dioxide, \({\text{Si}}{{\text{O}}_{\text{2}}}\)?
A. Each silicon atom forms four single covalent bonds to oxygen atoms.
B. Each silicon atom forms two double covalent bonds to oxygen atoms.
C. Each silicon atom forms two single covalent bonds to oxygen atoms.
D. Each silicon atom forms four double covalent bonds to oxygen atoms.
Markscheme
A
Examiners report
This was the question that caused candidates the most trouble, with a difficulty index of 5%. It would appear from the responses that about 80% of the candidates believe that the structure of silicon dioxide is identical to that of carbon dioxide. The discrimination index, at 0.04, was very low and this would seem to indicate that many candidates are not being made aware of these important structural differences.
Which species contain a dative covalent bond?
I. HCHO
II. CO
III. \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
This question asked which species contain a dative covalent bond from a list of three. One respondent stated that the term coordinate bond is often used, which is correct. However, in the guide in the teachers note corresponding to AS 4.2.2, the term that is used is dative covalent; hence candidates should be familiar with this term when used in questions. 40% of candidates gave the correct answer to this question.
Which molecule is polar?
A. \({\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\)
B. \({\text{BC}}{{\text{l}}_{\text{3}}}\)
C. \({\text{C}}{{\text{l}}_{\text{2}}}\)
D. \({\text{CC}}{{\text{l}}_{\text{4}}}\)
Markscheme
A
Examiners report
Which row correctly describes the bonding type and melting point of carbon and carbon dioxide?
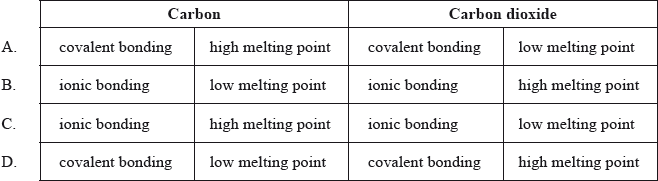
Markscheme
A
Examiners report
One G2 comment stated that none of the answers were correct for this question and stated that the question was not clear as there was no mention of intermolecular force considerations. The question itself simply involved looking at two features for both substances, carbon and carbon dioxide – firstly whether the bonding is ionic or covalent and secondly whether the melting point is high or low. It was not necessary to include intermolecular force considerations to answer this question, as clearly from the choices given A is the most appropriate answer. Clearly both carbon and carbon dioxide involve covalent bonding and carbon will involve a high melting point (particularly in the case of the allotropes, graphite and diamond, though of course the melting points of graphite and diamond are higher than that of fullerene) whereas the melting point for carbon dioxide will be low. 69% of candidates gave A as the correct answer.
Which species has the longest carbon to oxygen bond length?
A. CO
B. CH3OH
C. CH3CO2−
D. H2CO
Markscheme
B
Examiners report
Which compound has resonance structures?
A. C6H12
B. CH3CHO
C. NaBr
D. Na2CO3
Markscheme
D
Examiners report
Which statement is correct about carbon-oxygen bond lengths?
A. The C–O bond lengths are equal in propanoic acid, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{COOH}}\).
B. The C–O bond length in carbon dioxide, \({\text{C}}{{\text{O}}_{\text{2}}}\), is longer than the C–O bond length in methanol, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\).
C. The C–O bond length in carbon dioxide, \({\text{C}}{{\text{O}}_{\text{2}}}\), is longer than the C–O bond length in carbon monoxide, CO.
D. The C–O bond lengths are equal in ethyl ethanoate, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\).
Markscheme
C
Examiners report
What are the strongest intermolecular forces between molecules of propanone, CH3COCH3, in the liquid phase?
A. London (dispersion) forces
B. Covalent bonding
C. Hydrogen bonding
D. Dipole–dipole forces
Markscheme
D
Examiners report
Which of the following does not react with dilute HCl(aq)?
A. Na2CO3
B. Cu
C. Zn
D. CuO
Markscheme
B
Examiners report
Which correctly states the strongest intermolecular forces in the compounds below?
Markscheme
B
Examiners report
Which substance has a giant covalent structure?
Markscheme
D
Examiners report
Which combination describes the sulfate(IV) ion, SO32– (also known as sulfite ion)?
Markscheme
D