
HL Paper 3
The colour of olive oil is due to pigments such as chlorophyll, pheophytin and carotenoids.
The absorption spectrum of one form of pheophytin is shown below.
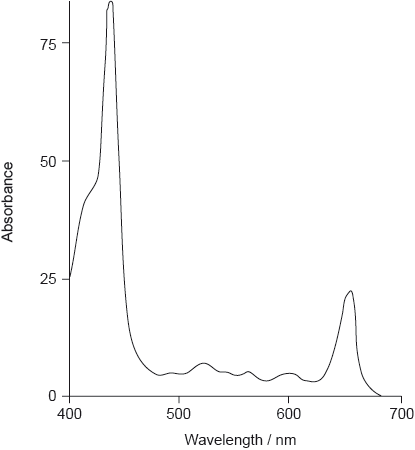
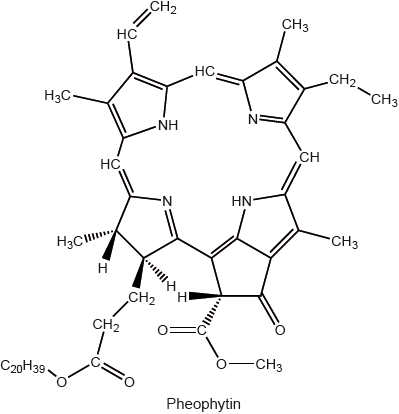
State the structural feature of a pheophytin molecule which allows it to absorb visible light.
Carotenoids may lose their colour and develop off odours when they are oxidized.
Identify, using table 22 of the data booklet, the structural feature that makes carotenoids susceptible to oxidation.
List two factors which increase the rate of oxidation of carotenoids.
Deduce, giving a reason, whether carotenoids are water-soluble or fat-soluble.
Markscheme
extensive conjugation/delocalization / extended system of conjugated double bonds/delocalized electrons;
Accept “contains porphyrin/porphin ring”.
multiple/conjugated C=C/carbon to carbon double bonds;
light;
higher/increased temperatures;
metals / transition metal ions;
hydroperoxides / peroxides;
Accept acidity/low pH.
fat-soluble and many non-polar groups/long non-polar chain;
Accept fat-soluble and absence of hydroxyl/OH/polar/H-bonding groups.
Examiners report
Though appreciation of the issue of complementary colours was widespread, hardly any candidates identified both absorption bands in the visible region. In the next few parts of the question many students lost marks by failing to answer fully, not referring to the extensive conjugation or the large number of carbon-carbon double bonds, as well as referring to temperature, rather than increased temperature, as a factor in the rate of oxidation. Lack of polarity was widely given as a reason for the fat solubility of carotenoids, though lack of groups able to form hydrogen bonds might have been more accurate (many quite polar molecules, such as \({\text{CHC}}{{\text{l}}_{\text{3}}}\), are insoluble in water). The features that lead to phospholipids acting as emulsifiers were often known, but again students tended to lose marks because their answers did not contain enough detail of their interaction between the phases present.
Though appreciation of the issue of complementary colours was widespread, hardly any candidates identified both absorption bands in the visible region. In the next few parts of the question many students lost marks by failing to answer fully, not referring to the extensive conjugation or the large number of carbon-carbon double bonds, as well as referring to temperature, rather than increased temperature, as a factor in the rate of oxidation. Lack of polarity was widely given as a reason for the fat solubility of carotenoids, though lack of groups able to form hydrogen bonds might have been more accurate (many quite polar molecules, such as \({\text{CHC}}{{\text{l}}_{\text{3}}}\), are insoluble in water). The features that lead to phospholipids acting as emulsifiers were often known, but again students tended to lose marks because their answers did not contain enough detail of their interaction between the phases present.
Though appreciation of the issue of complementary colours was widespread, hardly any candidates identified both absorption bands in the visible region. In the next few parts of the question many students lost marks by failing to answer fully, not referring to the extensive conjugation or the large number of carbon-carbon double bonds, as well as referring to temperature, rather than increased temperature, as a factor in the rate of oxidation. Lack of polarity was widely given as a reason for the fat solubility of carotenoids, though lack of groups able to form hydrogen bonds might have been more accurate (many quite polar molecules, such as \({\text{CHC}}{{\text{l}}_{\text{3}}}\), are insoluble in water). The features that lead to phospholipids acting as emulsifiers were often known, but again students tended to lose marks because their answers did not contain enough detail of their interaction between the phases present.
Though appreciation of the issue of complementary colours was widespread, hardly any candidates identified both absorption bands in the visible region. In the next few parts of the question many students lost marks by failing to answer fully, not referring to the extensive conjugation or the large number of carbon-carbon double bonds, as well as referring to temperature, rather than increased temperature, as a factor in the rate of oxidation. Lack of polarity was widely given as a reason for the fat solubility of carotenoids, though lack of groups able to form hydrogen bonds might have been more accurate (many quite polar molecules, such as \({\text{CHC}}{{\text{l}}_{\text{3}}}\), are insoluble in water). The features that lead to phospholipids acting as emulsifiers were often known, but again students tended to lose marks because their answers did not contain enough detail of their interaction between the phases present.
Nuclear reactions transform one nuclide into another. Fission, splitting a large nucleus into two smaller nuclei, releases vast amounts of energy.
(i) Uranium hexafluoride, UF6, is used in the uranium enrichment process that produces fuel for nuclear reactors.
State the molecular shape of uranium hexafluoride.
(ii) Explain why uranium dioxide, UO2, has a very high melting point whereas uranium hexafluoride vapourises easily into gas.
Markscheme
i
octahedral
Accept “square bipyramidal”
ii
UO2 strong bonding throughout crystal structure
UF6 molecular «covalent bonds between atoms» AND London/dispersion/instantaneous induced dipole-induced dipole forces between molecules
Accept “UO2 has ionic lattice”
Examiners report
The oxygen levels in water can change for a number of reasons.
The use of phosphate fertilizers can also produce changes in the oxygen concentrations in a river.
Phosphate ions can be removed from a solution by adding calcium ions. State the ionic equation for the reaction of calcium ions with phosphate ions.
Deduce the expression for the solubility product constant, \({K_{{\text{sp}}}}\), of calcium phosphate.
The solubility product of calcium phosphate is \({\text{2.07}} \times {\text{1}}{{\text{0}}^{ - 33}}\) at 298 K. Determine the concentration, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), of calcium ions, \({\text{C}}{{\text{a}}^{2 + }}\), in a saturated aqueous solution of calcium phosphate.
Markscheme
\({\text{3C}}{{\text{a}}^{{\text{2 + }}}}{\text{(aq)}} + {\text{2PO}}_{\text{4}}^{3 - }{\text{(aq)}} \rightleftharpoons {\text{C}}{{\text{a}}_{\text{3}}}{{\text{(P}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{2}}}{\text{(s)}}\);
Ignore state symbols.
Accept single arrow sign.
\({\text{(}}{K_{{\text{sp}}}} = {\text{) [C}}{{\text{a}}^{2 + }}{{\text{]}}^3}{{\text{[PO}}_4^{3 - }{\text{]}}^2}\);
Ignore state symbols.
Do not award mark if incorrect brackets are used or are missing.
Let x be solubility so \(2.07 \times {10^{ - 33}} = {(3x)^3}{(2x)^2}\);
Remember to apply ECF from (ii).
\({x^5} = \frac{{2.07 \times {{10}^{ - 33}}}}{{(27 \times 4)}}/1.92 \times {10^{ - 35}}/x = 1.14 \times {10^{ - 7}}\);
\(\left( {[{\text{C}}{{\text{a}}^{2 + }}] = 3x = } \right){\text{ }}3.42 \times {10^{ - 7}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{)}}\);
Award [3] for final correct answer.
Examiners report
Q was only identified by the stronger candidates and even then few stated that waste needs oxygen to decompose. The ionic equation for the reaction of calcium ions with phosphate ions proved a real minefield. It was highly disappointing at HL that so many candidates did not know what the formula and charge of the phosphate anion actually is. Some gave phosphite and several gave phosphide. Core chemistry underpins all options and candidates need to be prepared to apply some basic chemical principles to the various topics in the options. This aspect will be further enhanced in the new chemistry syllabus, but performance of candidates in this particular question shows the importance of this even in the current syllabus. In (ii), candidates often used the ionic equation in (i) to write the solubility product expression and hence had an incorrect inverse equation. Many also did not realize that the activity of a species in the solid state is unity. In (iii), incorrect \({K_{{\text{sp}}}}\) expressions in (ii) threw some candidates and others could not deal with the math involved in the solution to the equation. Many thought the final answer was x and not 3x for \({\text{[C}}{{\text{a}}^{2 + }}{\text{]}}\). Of course the stronger candidates scored all three marks on this question. In (c), misreading of the question was common which specifically asked for a non-chemical reason for the decrease in oxygen concentration i.e. an increase in the temperature of the water.
Q was only identified by the stronger candidates and even then few stated that waste needs oxygen to decompose. The ionic equation for the reaction of calcium ions with phosphate ions proved a real minefield. It was highly disappointing at HL that so many candidates did not know what the formula and charge of the phosphate anion actually is. Some gave phosphite and several gave phosphide. Core chemistry underpins all options and candidates need to be prepared to apply some basic chemical principles to the various topics in the options. This aspect will be further enhanced in the new chemistry syllabus, but performance of candidates in this particular question shows the importance of this even in the current syllabus. In (ii), candidates often used the ionic equation in (i) to write the solubility product expression and hence had an incorrect inverse equation. Many also did not realize that the activity of a species in the solid state is unity. In (iii), incorrect \({K_{{\text{sp}}}}\) expressions in (ii) threw some candidates and others could not deal with the math involved in the solution to the equation. Many thought the final answer was x and not 3x for \({\text{[C}}{{\text{a}}^{2 + }}{\text{]}}\). Of course the stronger candidates scored all three marks on this question. In (c), misreading of the question was common which specifically asked for a non-chemical reason for the decrease in oxygen concentration i.e. an increase in the temperature of the water.
Q was only identified by the stronger candidates and even then few stated that waste needs oxygen to decompose. The ionic equation for the reaction of calcium ions with phosphate ions proved a real minefield. It was highly disappointing at HL that so many candidates did not know what the formula and charge of the phosphate anion actually is. Some gave phosphite and several gave phosphide. Core chemistry underpins all options and candidates need to be prepared to apply some basic chemical principles to the various topics in the options. This aspect will be further enhanced in the new chemistry syllabus, but performance of candidates in this particular question shows the importance of this even in the current syllabus. In (ii), candidates often used the ionic equation in (i) to write the solubility product expression and hence had an incorrect inverse equation. Many also did not realize that the activity of a species in the solid state is unity. In (iii), incorrect \({K_{{\text{sp}}}}\) expressions in (ii) threw some candidates and others could not deal with the math involved in the solution to the equation. Many thought the final answer was x and not 3x for \({\text{[C}}{{\text{a}}^{2 + }}{\text{]}}\). Of course the stronger candidates scored all three marks on this question. In (c), misreading of the question was common which specifically asked for a non-chemical reason for the decrease in oxygen concentration i.e. an increase in the temperature of the water.
Low density polyethene (LDPE) and high density polyethene (HDPE) are both addition polymers.
Describe how the monomers of addition polymers and of condensation polymers differ.
Identify the type of intermolecular bonding that is responsible for Kevlar®’s strength.
Markscheme
addition: C=C
AND
condensation: two functional groups needed on each monomer
Accept "alkene/alkenyl" OR "double bond" OR "multiple bond".
hydrogen bonds
Accept “\(\pi - \pi \) stacking/interactions”.