
HL Paper 3
The colour of olive oil is due to pigments such as chlorophyll, pheophytin and carotenoids.
The absorption spectrum of one form of pheophytin is shown below.
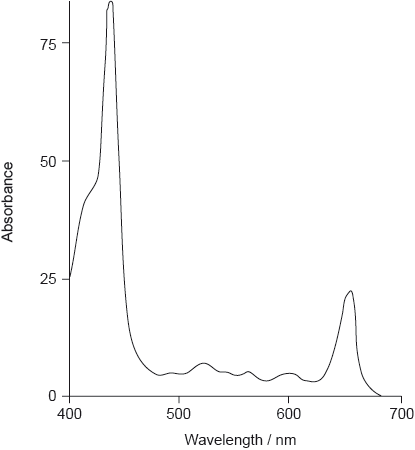
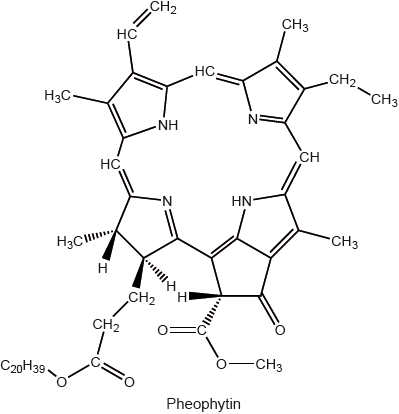
State the structural feature of a pheophytin molecule which allows it to absorb visible light.
Carotenoids may lose their colour and develop off odours when they are oxidized.
Identify, using table 22 of the data booklet, the structural feature that makes carotenoids susceptible to oxidation.
List two factors which increase the rate of oxidation of carotenoids.
Deduce, giving a reason, whether carotenoids are water-soluble or fat-soluble.
Nuclear reactions transform one nuclide into another. Fission, splitting a large nucleus into two smaller nuclei, releases vast amounts of energy.
(i) Uranium hexafluoride, UF6, is used in the uranium enrichment process that produces fuel for nuclear reactors.
State the molecular shape of uranium hexafluoride.
(ii) Explain why uranium dioxide, UO2, has a very high melting point whereas uranium hexafluoride vapourises easily into gas.
The oxygen levels in water can change for a number of reasons.
The use of phosphate fertilizers can also produce changes in the oxygen concentrations in a river.
Phosphate ions can be removed from a solution by adding calcium ions. State the ionic equation for the reaction of calcium ions with phosphate ions.
Deduce the expression for the solubility product constant, \({K_{{\text{sp}}}}\), of calcium phosphate.
The solubility product of calcium phosphate is \({\text{2.07}} \times {\text{1}}{{\text{0}}^{ - 33}}\) at 298 K. Determine the concentration, in \({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\), of calcium ions, \({\text{C}}{{\text{a}}^{2 + }}\), in a saturated aqueous solution of calcium phosphate.
Low density polyethene (LDPE) and high density polyethene (HDPE) are both addition polymers.
Describe how the monomers of addition polymers and of condensation polymers differ.
Identify the type of intermolecular bonding that is responsible for Kevlar®’s strength.