
HL Paper 2
This question is about the reactions of halogenoalkanes.
Compare and contrast the mechanisms by which 1-chlorobutane, CH3CH2CH2CH2Cl, and 2-chloro-2-methylpropane, (CH3)3CCl, react with aqueous sodium hydroxide, giving two similarities and one difference.
Outline why the rate of reaction of the similar bromo-compounds is faster.
State the organic product of the reaction between 1-chlorobutane, CH3CH2CH2CH2Cl, and aqueous sodium hydroxide.
Suggest how this product could be synthesized in one step from butanoic acid.
Deduce the name of the class of compound formed when the product of (c)(i) reacts with butanoic acid.
Geometrical isomerism and optical isomerism are two sub-groups of stereoisomerism in organic chemistry.
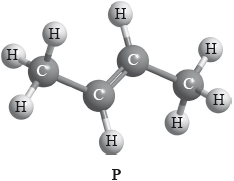
Compound P has the following three-dimensional structure. P also has geometrical isomers.
Menthol can be used in cough medicines. The compound contains C, H and O only.
Describe what is meant by the term stereoisomers.
Geometrical isomers have different physical properties and many drugs, such as doxepin (which has antidepressant properties), have geometrical isomers.
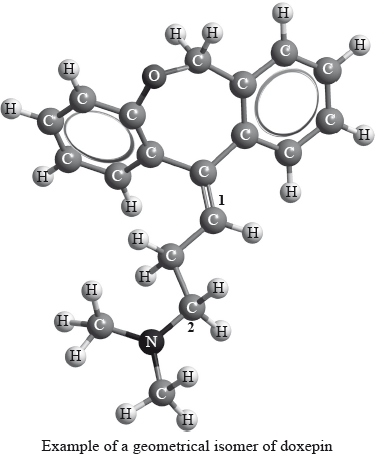
For each of the carbon atoms labelled 1 and 2 in doxepin, deduce the type of hybridization involved (sp, sp2 or sp3).
1:
2:
Clomifene, a fertility drug, whose three-dimensional structure is represented below, also has geometrical isomers.
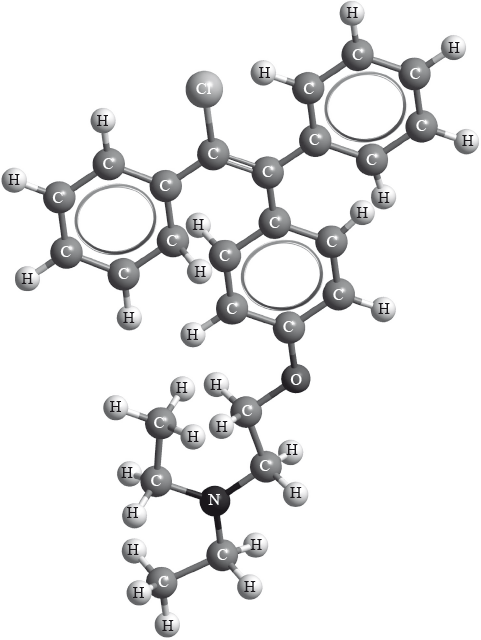
Identify the name of one functional group present in clomifene.
Draw any two other isomers of P.
Apply IUPAC rules to state the names of all the straight-chain isomers of compounds of molecular formula C4H8 (including P).
State the structural formula of the organic products, Q, R, S and T, formed in the following reactions.
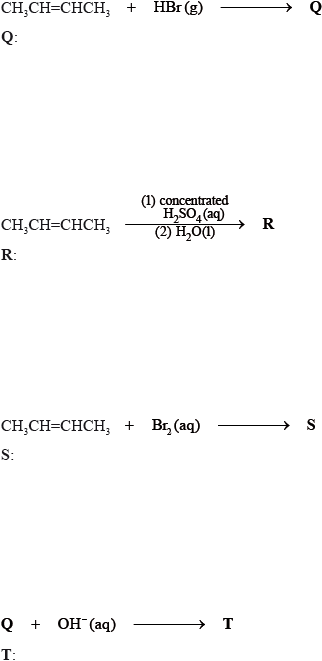
Suggest one suitable mechanism for the reaction of Q with aqueous sodium hydroxide to form T, using curly arrows to represent the movement of electron pairs.
State the structural formula of the organic product formed, U, when R is heated under reflux with acidified potassium dichromate(VI).
Apply IUPAC rules to state the name of this product, U.
When a \(6.234 \times {10^{ - 2}}{\text{ g}}\) of the compound was combusted, \(1.755 \times {10^{ - 1}}{\text{ g}}\) of carbon dioxide and \(7.187 \times {10^{ - 2}}{\text{ g}}\) of water were produced. Determine the molecular formula of the compound showing your working, given that its molar mass is \(M = 156.30{\text{ g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
Menthol occurs naturally and has several isomers. State the structural feature of menthol which is responsible for it having enantiomers.
State the instrument used to distinguish between each of the two enantiomers, and how they could be distinguished using this instrument.
Compare the physical and chemical properties of enantiomers.
Physical properties:
Chemical properties:
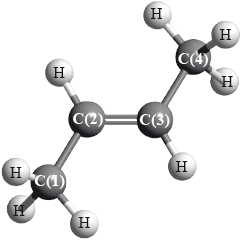
But-2-ene is a straight-chain alkene with formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}\). The molecule contains both \(\sigma \) and \(\pi \) bonds.
The polymerization of the alkenes is one of the most significant reactions of the twentieth century.
(i) Explain the formation of the \(\pi \) bond.
(ii) For each of the carbon atoms, C(1) and C(2), identify the type of hybridization shown.
C(1):
C(2):
But-2-ene shows geometrical isomerism. Draw the structural formula and state the name of the other geometrical isomer.
Identify the structural formula of an isomer of but-2-ene which does not decolourize bromine water, Br2(aq).
(i) Outline two reasons why the polymers of the alkenes are of economic importance.
(ii) State the type of polymerization reaction shown by the alkene in part (a).
(iii) Deduce the structure of the resulting polymer showing three repeating units.
(iv) Explain why monomers are often gases or volatile liquids, but polymers are solids.
But-2-ene belongs to the homologous series of the alkenes.
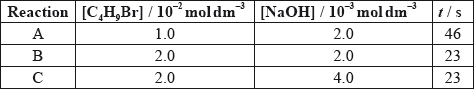
The time taken to produce a certain amount of product using different initial concentrations of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) and NaOH is measured. The results are shown in the following table.
Outline three features of a homologous series.
Describe a test to distinguish but-2-ene from butane, including what is observed in each case.
2-bromobutane can be produced from but-2-ene. State the equation of this reaction using structural formulas.
State what is meant by the term stereoisomers.
Explain the existence of geometrical isomerism in but-2-ene.
Deduce the order of reaction with respect to \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) and NaOH, using the data above.
\({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\)
NaOH:
Deduce the rate expression.
Based on the rate expression obtained in (c) (ii) state the units of the rate constant, \(k\).
Halogenalkanes can react with NaOH via \({{\text{S}}_{\text{N}}}{\text{1}}\) and \({{\text{S}}_{\text{N}}}{\text{2}}\) type mechanisms. Explain why \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\) reacts via the mechanism described in (d) (i).
Identify the rate-determining step of this mechanism.
Existence of isomers leads to diversity of organic compounds.
(a) Describe what is meant by the term stereoisomers.
(b) 1,3-dichlorocyclobutane exists as geometrical isomers, a form of stereoisomers.
(i) Draw and name the two geometrical isomers of 1,3-dichlorocyclobutane.
(ii) Identify the isomer with the higher boiling point and explain your reasoning.
Below are four structural isomers with molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\). State the name of each of the isomers a, b, c and D.
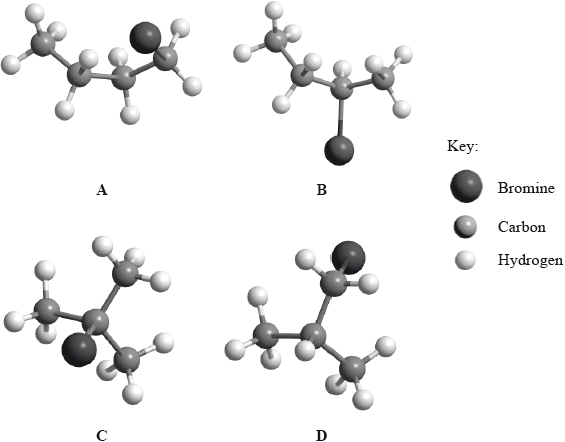
Identify the isomer(s) which will react with aqueous sodium hydroxide almost exclusively by an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. State the meaning of the symbols in the term \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism.
Using the formula RBr to represent a bromoalkane, state an equation for the rate determining step of this \({{\text{S}}_{\text{N}}}{\text{1}}\) reaction.
Identify one isomer that will react with aqueous sodium hydroxide almost exclusively by an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Draw the mechanism for this reaction using curly arrows to represent the movement of electron pairs. Include the structural formulas of the transition state and the organic product.
State and explain how the rates of the reactions in parts (b) (i) and (b) (iii) are affected when the concentration of the sodium hydroxide is doubled.
State and explain how the rate of reaction of 1-bromobutane with sodium hydroxide compares with that of 1-chlorobutane with sodium hydroxide.
Identify the isomer of C4H9Br that can exist as stereoisomers. Outline how a polarimeter will distinguish between the isomers, and how their physical and chemical properties compare.
Alkenes, alcohols and esters are three families of organic compounds with many commercial uses.
An ester which gives apples their characteristic smell contains C, H and O. When \(3.00 \times {10^{ - 3}}{\text{ g}}\) of this ester were completely combusted, \(6.93 \times {10^{ - 3}}{\text{ g}}\) of \({\text{C}}{{\text{O}}_{\text{2}}}\) and \(2.83 \times {10^{ - 3}}{\text{ g}}\) of \({{\text{H}}_{\text{2}}}{\text{O}}\) were produced.
State what is meant by the term stereoisomers.
Determine the empirical formula of the ester, showing your working.
The molar mass of the ester is \({\text{116.18 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\). Determine its molecular formula.
2-bromobutane is optically active. Draw the two enantiomers of 2-bromobutane and compare their physical and chemical properties.
2-methylbutan-2-ol, \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{C(OH)C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\), is a liquid with a smell of camphor that was formerly used as a sedative. One way of producing it starts with 2-methylbut-2-ene.
As well as 2-methylbutan-2-ol, the reaction also produces a small quantity of an optically active isomer, X.
2-methylbutan-2-ol can also be produced by the hydrolysis of 2-chloro-2-methylbutane, \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CCl}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\), with aqueous sodium hydroxide.
2-chloro-2-methylbutane contains some molecules with a molar mass of approximately \({\text{106 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) and some with a molar mass of approximately \({\text{108 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
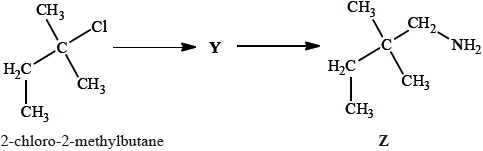
2-chloro-2-methylbutane can also be converted into compound Z by a two-stage reaction via compound Y:
State the other substances required to convert 2-methylbut-2-ene to 2-methylbutan-2-ol.
Explain whether you would expect 2-methylbutan-2-ol to react with acidified potassium dichromate(VI).
State what is meant by optical activity.
State what optical activity indicates about the structure of the molecule.
Optical activity can be detected using a polarimeter. Explain how this works.
Deduce the structural formula of X.
Explain why 2-methylbut-2-ene is less soluble in water than 2-methylbutan-2-ol.
Explain the mechanism of this reaction using curly arrows to represent the movement of electron pairs.
State the rate expression for this reaction and the units of the rate constant.
Suggest why, for some other halogenoalkanes, this hydrolysis is much more effective in alkaline rather than in neutral conditions.
Outline why there are molecules with different molar masses.
Draw the structure of Y.
State the reagent and any catalyst required for both the formation of Y and the conversion of Y into Z.
Formation of Y:
Conversion of Y into Z:
One structural isomer of C4H9Br is a chiral molecule.
Draw the three-dimensional shape of each enantiomer of this isomer showing their spatial relationship to each other.
When one enantiomer undergoes substitution by alkaline hydrolysis approximately 75 % of the product molecules show inversion of configuration. Comment on the mechanisms that occur.
Suggest why the rate of alkaline hydrolysis of an enantiomer of iodopropane is greater than that of an enantiomer of bromopropane.
In some countries, ethanol is mixed with gasoline (petrol) to produce a fuel for cars called gasohol.
Deduce a two-step synthesis for each of the following conversions. For each step, state the structural formulas of all reactants and products and state the conditions used in the reactions.
Ethanol to ethyl ethanoate.
Propene to propanone.
The reagents used in an elimination reaction are shown below.
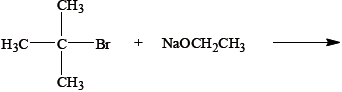
Explain the mechanism of this reaction using curly arrows to represent the movement of electron pairs.
Describe geometrical isomerism.
Draw the geometrical isomers of but-2-ene.
Draw the two enantiomers of butan-2-ol.
The compound \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{7}}}{\text{Cl}}\) can exhibit stereoisomerism.
The reaction between bromoethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\), and potassium cyanide is an example of a nucleophilic substitution reaction.
Draw the structural formulas of the two geometrical isomers of 1-chloro-but-2-ene.
Explain why 1-chloro-but-2-ene shows geometrical isomerism.
Draw the structural formula of one isomer of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{7}}}{\text{Cl}}\) that shows optical isomerism and identify the chiral carbon atom with an asterisk (*).
State whether this reaction is SN1 or SN2.
Explain the mechanism of the reaction using curly arrows to represent the movement of electron pairs.
The organic product obtained in part (c) (ii) can be reduced to form an amine. State an equation for the reaction, naming the catalyst involved.
But-1-ene and 1-aminobutane (1-butylamine) can both be prepared from 1-bromobutane.
2-bromobutane and 2-bromo-2-methylpropane are two isomers of 1-bromobutane.
Identify the type of reaction and explain the mechanism for the preparation of but-1-ene from 1-bromobutane using curly arrows to represent the movement of electron pairs.
State the equation (using structural formulas) for the preparation of 1-aminobutane from 1-bromobutane. State the necessary reagents and conditions of the reaction.
Explain the mechanism for the preparation of 1-aminobutane from 1-bromobutane using curly arrows to represent the movement of electron pairs.
Draw the structures of the two mirror images of the isomer that can exhibit optical isomerism.
Describe how the two optical isomers can be distinguished practically using plane-polarized light.
Explain why the mechanism of the reaction will be different if 1-bromobutane is replaced by 2-bromo-2-methylpropane to form 2-amino-2-methylpropane in the reaction in part (a) (iv).
Consider the two-stage reaction pathway below.
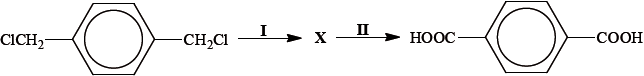
Deduce the structural formula of compound X.
State the reagents and conditions required for stage II of the pathway.
Reagents:
Conditions:
Consider the following reactions.
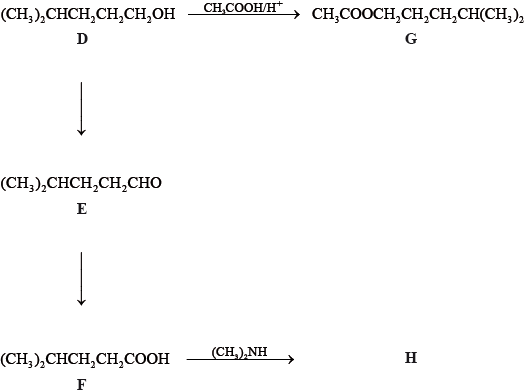
State the IUPAC names of each of the compounds, D, E, F and G.
D:
E:
F:
G:
State the reagents and reaction conditions used to convert D to E and D to F directly.
Discuss the volatility of E compared to F.
Some reactions of but-2-ene are given below.
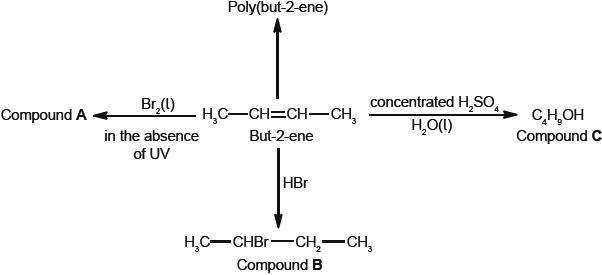
But-2-ene can exist as two geometrical isomers. Cis-trans is a form of stereoisomerism.
Deduce the full structural formula of compound A.
Apply IUPAC rules to name compound A.
Describe the colour change observed when excess but-2-ene reacts with bromine to form compound A.
(i) Outline two reasons why the polymerization of alkenes is of economic importance.
(ii) Identify the structure of the repeating unit of poly(but-2-ene).
Compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\), can also be formed by reacting compound B, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHBrC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\), with aqueous potassium hydroxide. This reaction proceeds by both \({{\text{S}}_{\text{N}}}{\text{1}}\) and \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanisms. Explain the \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism, using curly arrows to represent the movement of electron pairs.
Explain why the hydroxide ion is a better nucleophile than water.
Compound C, \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{OH}}\), can be oxidized by acidified potassium dichromate(VI) to form compound F.
(i) State the name of the functional group present in compound F.
(ii) Deduce the structural formula of an alcohol which is a structural isomer of compound C and cannot be oxidized by acidified potassium dichromate(VI).
Explain why but-2-ene is more volatile than compound C.
Deduce the equation for the complete combustion of compound C.
Define the term stereoisomers.
State the conditions needed for a compound to show cis-trans.
Draw the structures of the two geometrical isomers of but-2-ene, clearly identifying each as cis or trans.
In an experiment conducted at 25.0 °C, the initial concentration of propanoic acid and methanol were \({\text{1.6 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and \({\text{2.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) respectively. Once equilibrium was established, a sample of the mixture was removed and analysed. It was found to contain \({\text{0.80 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) of compound X.
Two compounds, A and D, each have the formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Cl}}\).
Compound A is reacted with dilute aqueous sodium hydroxide to produce compound B with a formula of \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\). Compound B is then oxidized with acidified potassium
manganate(VII) to produce compound C with a formula of \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{8}}}{\text{O}}\). Compound C resists further oxidation by acidified potassium manganate(VII).
Compound D is reacted with dilute aqueous sodium hydroxide to produce compound E with a formula of \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}{\text{O}}\). Compound E does not react with acidified potassium manganate(VII).
Deduce the structural formulas for compounds A, B, C, D and E.
A:
B:
C:
D:
E:
Deduce an equation for the reaction between propanoic acid and methanol. Identify the catalyst and state the name of the organic compound, X, formed.
Calculate the concentrations of the other three species present at equilibrium.
State the equilibrium constant expression, \({K_{\text{c}}}\), and calculate the equilibrium constant for this reaction at 25.0 °C.
2-chloro-3-methylbutane reacts with sodium hydroxide via an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Explain the mechanism by using curly arrows to represent the movement of electron pairs.
Explain why the hydroxide ion is a better nucleophile than water.
1-chlorobutane can be converted to a pentylamine via a two stage process. Deduce equations for each step of this conversion including any catalyst required and name the organic product produced at each stage.
There are several structural isomers with the molecular formula \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{Br}}\).
All the isomers react when warmed with a dilute aqueous solution of sodium hydroxide according to the equation below.
\[{{\text{C}}_5}{{\text{H}}_{11}}{\text{Br}} + {\text{NaOH}} \to {{\text{C}}_5}{{\text{H}}_{11}}{\text{OH}} + {\text{NaBr}}\]
Deduce the name of one of the isomers which can exist as enantiomers and draw three-dimensional representations of its two enantiomers.
The reaction with 1-bromopentane proceeds by an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism. Describe this mechanism using structural formulas and curly arrows to represent the movement of electron pairs.
The reaction with 2-bromo-2-methylbutane proceeds by an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. Describe this mechanism using structural formulas and curly arrows to represent the movement of electron pairs.
Explain why 1-bromopentane reacts by an \({{\text{S}}_{\text{N}}}{\text{2}}\) mechanism whereas 2-bromo-2-methylbutane reacts by an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism.
Explain whether the boiling point of 1-bromopentane will be higher, lower or the same as that of 2-bromo-2-methylbutane.
The product \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{11}}}}{\text{OH}}\) formed from the reaction with 1-bromopentane is warmed with ethanoic acid in the presence of a few drops of concentrated sulfuric acid. State the name of the type of reaction taking place and the structural formula of the organic product.
Iron rusts in the presence of oxygen and water. Rusting is a redox process involving several steps that produces hydrated iron(III) oxide, \({\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}} \bullet {\text{n}}{{\text{H}}_{\text{2}}}{\text{O}}\), as the final product.
The half-equations involved for the first step of rusting are given below.
Half-equation 1: \({\text{Fe(s)}} \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\)
Half-equation 2: \({{\text{O}}_{\text{2}}}{\text{(aq)}} + {\text{4}}{{\text{e}}^ - } + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4O}}{{\text{H}}^ - }{\text{(aq)}}\)
(i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason for your answer.
(ii) Identify the oxidation number of each atom in the three species in half-equation 2.
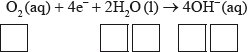
(iii) Deduce the overall redox equation for the first step of rusting by combining half-equations 1 and 2.
(iv) Identify the reducing agent in the redox equation in part (iii).
The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very small concentrations. Explain, in terms of intermolecular forces, why oxygen is not very soluble in water.
State the relationship between the electron arrangement of an element and its group and period in the periodic table.
Transition metals and their compounds often catalyse reactions. The catalyzed decomposition of hydrogen peroxide by CuO is an example. State two other examples of catalyzed reactions giving the transition metal or its compound acting as catalyst.
(i) State a chemical equation for the partial dissociation of water into ions, including state symbols.
(ii) The dissociation of water into ions is reversible. State the expression for the ionic product constant of water.
(iii) The ionic product constant of water was measured at three different temperatures.
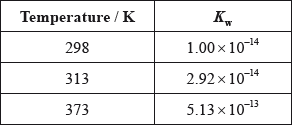
Deduce whether the ionization of water is exothermic or endothermic, giving your reason.
(iv) Use the data in part (iii) to determine the pH of water at 373 K, correct to two decimal places.
(i) An aqueous solution of sodium chloride is electrolysed using inert electrodes. Explain which product is obtained at the positive electrode (anode) if the concentration of sodium chloride is high.
(ii) State the half-equations occurring at the electrodes during the electrolysis of the concentrated aqueous solution of sodium chloride.
Negative electrode (cathode):
Positive electrode (anode):
Describe how electrolysis can be used to electroplate a bracelet with a layer of silver metal. Include the choice of electrodes and electrolyte needed in your description.
Ethanol is a primary alcohol that can be oxidized by acidified potassium dichromate(VI). Distinguish between the reaction conditions needed to produce ethanal and ethanoic acid.
Ethanal:
Ethanoic acid:
Determine the oxidation number of carbon in ethanol and ethanal.
Ethanol:
Ethanal:
Deduce the half-equation for the oxidation of ethanol to ethanal.
Deduce the overall redox equation for the reaction of ethanol to ethanal with acidified potassium dichromate(VI).
Ethanol can be made by reacting aqueous sodium hydroxide with bromoethane.
Explain the mechanism for this reaction, using curly arrows to represent the movement of electron pairs.
Determine the orders of reaction of the reactants and the overall rate expression for the reaction between 2-bromobutane and aqueous sodium hydroxide using the data in the table.
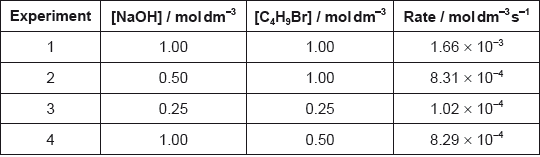
Determine the rate constant, \(k\), with its units, using the data from experiment 3.
Identify the molecularity of the rate-determining step in this reaction.
2-bromobutane exists as optical isomers.
State the essential feature of optical isomers.
2-bromobutane exists as optical isomers.
Outline how a polarimeter can distinguish between these isomers.
Describe the formation of \(\sigma \) and \(\pi \) bonds in an alkene.
The two most abundant isotopes of bromine have the mass numbers 79 and 81.
Calculate the relative abundance of \(^{{\text{79}}}{\text{Br}}\) using table 5 of the data booklet, assuming the abundance of the other isotopes is negligible.
Consider the following sequence of reactions.
\[{\text{RC}}{{\text{H}}_3}\xrightarrow{{reaction 1}}{\text{RC}}{{\text{H}}_2}{\text{Br}}\xrightarrow{{reaction 2}}{\text{RC}}{{\text{H}}_2}{\text{OH}}\]
\({\text{RC}}{{\text{H}}_{\text{3}}}\) is an unknown alkane in which R represents an alkyl group.
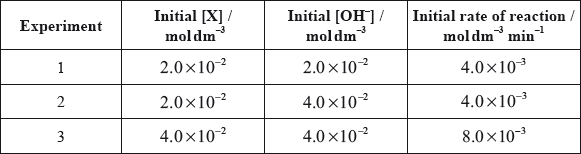
All the isomers can by hydrolysed with aqueous sodium hydroxide solution. When the reaction of one of these isomers, X, was investigated the following kinetic data were obtained.
The alkane contains 82.6% by mass of carbon. Determine its empirical formula, showing your working.
A 1.00 g gaseous sample of the alkane has a volume of 385 cm3 at standard temperature and pressure. Deduce its molecular formula.
State the reagent and conditions needed for reaction 1.
Reaction 1 involves a free-radical mechanism. Describe the stepwise mechanism, by giving equations to represent the initiation, propagation and termination steps.
The mechanism in reaction 2 is described as SN2. Explain the mechanism of this reaction using curly arrows to show the movement of electron pairs, and draw the structure of the transition state.
There are four structural isomers with the molecular formula \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}{\text{Br}}\). One of these structural isomers exists as two optical isomers. Draw diagrams to represent the three-dimensional structures of the two optical isomers.
(i) Deduce the rate expression for the reaction.
(ii) Determine the value of the rate constant for the reaction and state its units.
(iii) State the name of isomer X and explain your choice.
(iv) State equations for the steps that take place in the mechanism of this reaction and state which of the steps is slow and which is fast.
The reactivity of organic compounds depends on the nature and positions of their functional groups.
The structural formulas of two organic compounds are shown below.
Deduce, giving a reason, which of the two compounds can show optical activity.
Draw three-dimensional representations of the two enantiomers.
State the reagents used in the nitration of benzene.
State an equation for the formation of NO2+.
Explain the mechanism of the reaction between 2-bromo-2-methylpropane, (CH3)3CBr, and aqueous sodium hydroxide, NaOH (aq), using curly arrows to represent the movement of electron pairs.
Organic compounds often have isomers.
A straight chain molecule of formula C5H10O contains a carbonyl group. The compound cannot be oxidized by acidified potassium dichromate(VI) solution.
A tertiary halogenoalkane with three different alkyl groups, (R1R2R3)C−X, undergoes a SN1 reaction and forms two isomers.
Deduce the structural formulas of the two possible isomers.
Mass spectra A and B of the two isomers are given.
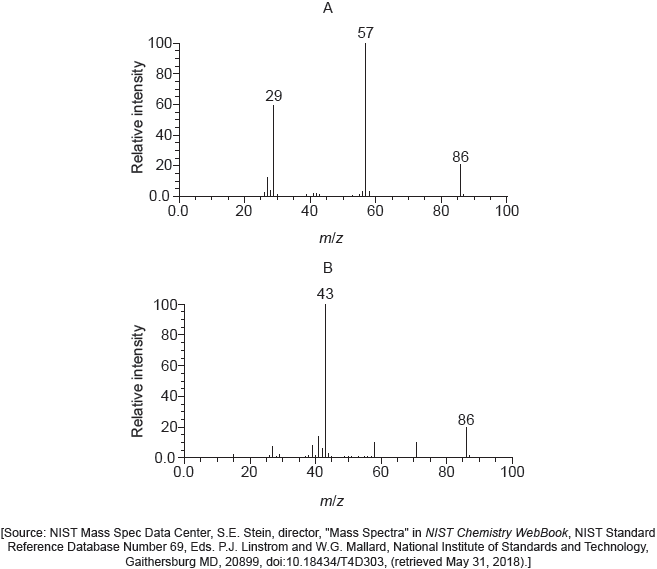
Explain which spectrum is produced by each compound using section 28 of the data booklet.
State the type of bond fission that takes place in a SN1 reaction.
State the type of solvent most suitable for the reaction.
Draw the structure of the intermediate formed stating its shape.
Suggest, giving a reason, the percentage of each isomer from the SN1 reaction.
Nitrobenzene, C6H5NO2, can be converted to phenylamine via a two-stage reaction.
In the first stage, nitrobenzene is reduced with tin in an acidic solution to form an intermediate ion and tin(II) ions. In the second stage, the intermediate ion is converted to phenylamine in the presence of hydroxide ions.
Formulate the equation for each stage of the reaction.
Benzene is an aromatic hydrocarbon.
Discuss the physical evidence for the structure of benzene.
State the typical reactions that benzene and cyclohexene undergo with bromine.
State the reagents used to convert benzene to nitrobenzene and the formula of the electrophile formed.
Explain the mechanism for the nitration of benzene, using curly arrows to show the movement of electron pairs.
State the reagents used in the two-stage conversion of nitrobenzene to aniline.
Bromomethane was used as a pesticide until it was found to be ozone-depleting.
State the equation for the reaction between methane and bromine to form bromomethane.
Explain, using equations, the complete free-radical mechanism for the reaction of methane with bromine, including necessary reaction conditions.
Bromomethane reacts with aqueous sodium hydroxide. State the organic product of this reaction.
Explain why the rate of the reaction between iodomethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{I}}\), and NaOH(aq) is faster than the rate of the reaction between \({\text{C}}{{\text{H}}_{\text{3}}}{\text{Br}}\) and NaOH(aq).
Bromine can be produced by the electrolysis of molten sodium bromide.
Deduce the half-equation for the reaction at each electrode.
Positive electrode (anode):
Negative electrode (cathode):
Predict the products formed at the electrodes during the electrolysis of concentrated aqueous sodium bromide.
Positive electrode (anode):
Negative electrode (cathode):
Bromine reacts with aqueous sodium iodide.
\[{\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}} + {\text{2NaI(aq)}} \to {{\text{I}}_{\text{2}}}{\text{(aq)}} + {\text{2NaBr(aq)}}\]
Identify the oxidizing agent in this reaction.
Define the term standard electrode potential, \({E^\Theta }\).
Draw a labelled diagram for the voltaic cell in which the following reaction occurs.
\[{\text{Mg(s)}} + {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{Cu(s)}}\]
Include in your answer the direction of electron flow and the polarity of the electrodes.
A student measures a voltage of 2.65 V in the voltaic cell formed between magnesium and copper half-cells using a digital voltmeter.
State the random uncertainty of this value, in V, and the number of significant figures in the answer.
Random uncertainty:
Significant figures:
Outline how the student can reduce the random error in her results.
Determine the standard enthalpy change of formation, \(\Delta H_{\text{f}}^\Theta \), of NaCl(s), in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\), using a Born-Haber cycle and tables 7, 10 and 13 of the data booklet. The standard enthalpy change of atomization (standard enthalpy change of sublimation), \(\Delta H_{{\text{at}}}^\Theta \), of Na(s) is \( + {\text{108 kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
\({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) of \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) ethanoic acid was added to \({\text{30.0 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.150 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) sodium hydrogencarbonate solution, \({\text{NaHC}}{{\text{O}}_{\text{3}}}{\text{(aq)}}\).
The molar mass of a volatile organic liquid, X, can be determined experimentally by allowing it to vaporize completely at a controlled temperature and pressure. 0.348 g of X was injected into a gas syringe maintained at a temperature of 90 °C and a pressure of \(1.01 \times {10^5}{\text{ Pa}}\). Once it had reached equilibrium, the gas volume was measured as \({\text{95.0 c}}{{\text{m}}^{\text{3}}}\).
Bromoethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\), undergoes a substitution reaction to form ethylamine, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\).
Many organic compounds exist as stereoisomers.
Outline how electrical conductivity can be used to distinguish between a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of ethanoic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\), and a \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of hydrochloric acid, HCl.
(i) State an equation for the reaction of ethanoic acid with a solution of sodium hydrogencarbonate.
(ii) Determine which is the limiting reagent. Show your working.
(iii) Calculate the mass, in g, of carbon dioxide gas produced.
(i) Determine the amount, in mol, of X in the gas syringe.
(ii) Calculate the molar mass of X.
Deduce the mechanism for the reaction using equations and curly arrows to represent the movement of electron pairs.
(i) Outline the meaning of the term stereoisomers.
(ii) Draw the structures of the two stereoisomers of dichloroethene, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
(iii) Explain why this type of stereoisomerism exists in \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).
(iv) Draw the structures of the two stereoisomers of 1-chloro-1-fluoroethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{FCl}}\), showing the relationship between them.
(v) Outline how the two isomers of \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{FCl}}\) could be distinguished from each other.
This question is about carbon and chlorine compounds.
Ethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\), reacts with chlorine in sunlight. State the type of this reaction and the name of the mechanism by which it occurs.
Formulate equations for the two propagation steps and one termination step in the formation of chloroethane from ethane.
Deduce the splitting patterns in the 1H NMR spectrum of C2H5Cl.
Explain why tetramethylsilane (TMS) is often used as a reference standard in 1H NMR.
One possible product, X, of the reaction of ethane with chlorine has the following composition by mass:
carbon: 24.27%, hydrogen: 4.08%, chlorine: 71.65%
Determine the empirical formula of the product.
The mass and 1H NMR spectra of product X are shown below. Deduce, giving your reasons, its structural formula and hence the name of the compound.
When the product X is reacted with NaOH in a hot alcoholic solution, C2H3Cl is formed. State the role of the reactant NaOH other than as a nucleophile.
Chloroethene, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{\text{Cl}}\), can undergo polymerization. Draw a section of the polymer with three repeating units.
Propane and propene are members of different homologous series.
(i) Draw diagrams to show how sigma (σ) and pi (π) bonds are formed between atoms.
(ii) State the number of sigma (σ) and pi (π) bonds in propane and propene.
Construct the mechanism of the formation of 2-bromopropane from hydrogen bromide and propene using curly arrows to denote the movement of electrons.
A compound with a molecular formula C7H14O produced the following high resolution 1H NMR spectrum.
Deduce what information can be obtained from the 1H NMR spectrum.
Identify the functional group that shows stretching at 1710 cm–1 in the infrared spectrum of this compound using section 26 of the data booklet and the 1H NMR.
Suggest the structural formula of this compound.
Bromine was added to hexane, hex-1-ene and benzene. Identify the compound(s) which will react with bromine in a well-lit laboratory.
Deduce the structural formula of the main organic product when hex-1-ene reacts with hydrogen bromide.
State the reagents and the name of the mechanism for the nitration of benzene.
Outline, in terms of the bonding present, why the reaction conditions of halogenation are different for alkanes and benzene.
Below are two isomers, A and B, with the molecular formula C4H9Br.
Explain the mechanism of the nucleophilic substitution reaction with NaOH(aq) for the isomer that reacts almost exclusively by an SN2 mechanism using curly arrows to represent the movement of electron pairs.
Phosgene, COCl2, is usually produced by the reaction between carbon monoxide and chlorine according to the equation:
(i) Deduce the equilibrium constant expression, Kc, for this reaction.
(ii) At exactly 600°C the value of the equilibrium constant is 0.200. Calculate the standard Gibbs free energy change, , for the reaction, in kJ, using sections 1 and 2 of the data booklet. State your answer to three significant figures.
(iii) The standard enthalpy change of formation of phosgene, \(\Delta H_f^\Theta \), is −220.1kJmol−1. Determine the standard enthalpy change, \(\Delta H_{}^\Theta \), for the forward reaction of the equilibrium, in kJ, using section 12 of the data booklet.
(iv) Calculate the standard entropy change, \(\Delta S_{}^\Theta \), in JK−1, for the forward reaction at 25°C, using your answers to (a) (ii) and (a) (iii). (If you did not obtain an answer to (a) (ii) and/or (a) (iii) use values of +20.0 kJ and −120.0 kJ respectively, although these are not the correct answers.)
One important industrial use of phosgene is the production of polyurethanes. Phosgene is reacted with diamine X, derived from phenylamine.
(i) Classify diamine X as a primary, secondary or tertiary amine.
(ii) Phenylamine, C6H5NH2, is produced by the reduction of nitrobenzene, C6H5NO2. Suggest how this conversion can be carried out.
(iii) Nitrobenzene can be obtained by nitrating benzene using a mixture of concentrated nitric and sulfuric acids. Formulate the equation for the equilibrium established when these two acids are mixed.
(iv) Deduce the mechanism for the nitration of benzene, using curly arrows to indicate the movement of electron pairs.
The other monomer used in the production of polyurethane is compound Z shown below.
(i) State the name, applying IUPAC rules, of compound Z and the class of compounds to which it belongs.
Name:
Class:
(ii) Deduce the number of signals you would expect to find in the 1H NMR spectrum of compound Z, giving your reasons.
The mass spectrum and infrared (IR) spectrum of compound Z are shown below:
Mass spectrum
IR spectrum
(iii) Identify the species causing the large peak at m/z=31 in the mass spectrum.
(iv) Identify the bond that produces the peak labelled Q on the IR spectrum, using section 26 of the data booklet.
Phenylamine can act as a weak base. Calculate the pH of a 0.0100 mol dm−3 solution of phenylamine at 298K using section 21 of the data booklet.