
HL Paper 1
Which quantities are the same for all atoms of chlorine?
I. Number of protons
II. Number of neutrons
III. Number of electrons
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
The table below shows the number of protons, neutrons and electrons present in five species.
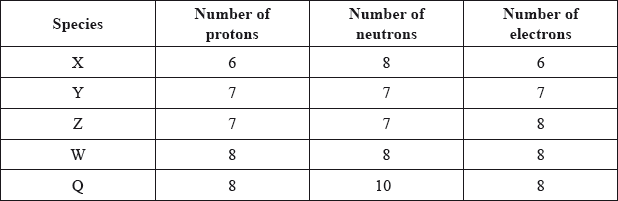
Which two species are isotopes of the same element?
A. X and W
B. Y and Z
C. Z and W
D. W and Q
What is the order of increasing energy of the orbitals within a single energy level?
A. \({\text{d}} < {\text{s}} < {\text{f}} < {\text{p}}\)
B. \({\text{s}} < {\text{p}} < {\text{d}} < {\text{f}}\)
C. \({\text{p}} < {\text{s}} < {\text{f}} < {\text{d}}\)
D. \({\text{f}} < {\text{d}} < {\text{p}} < {\text{s}}\)
Which species possesses only two unpaired electrons?
A. Zn
B. Mg
C. \({\text{T}}{{\text{i}}^{2 + }}\)
D. \({\text{F}}{{\text{e}}^{2 + }}\)
Which species has the electron configuration of \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{8}}}\)?
A. Ni
B. \({\text{N}}{{\text{i}}^{2 + }}\)
C. Fe
D. \({\text{C}}{{\text{u}}^{2 + }}\)
Which representation would be correct for a species, Z, which has 31 protons, 40 neutrons and 28 electrons?
A. \({}_{31}^{71}{{\rm{Z}}^{3 + }}\)
B. \({}_{31}^{71}{{\rm{Z}}^{3 - }}\)
C. \({}_{40}^{71}{{\rm{Z}}^{3 + }}\)
D. \({}_{28}^{71}{{\rm{Z}}^{3 + }}\)
What is the correct electron configuration of the \({\text{C}}{{\text{u}}^ + }\) ion?
A. \({\text{[Ar] 3}}{{\text{d}}^{\text{9}}}{\text{ 4}}{{\text{s}}^{\text{1}}}\)
B. \({\text{[Ar] 3}}{{\text{d}}^{\text{7}}}{\text{ 4}}{{\text{s}}^{\text{2}}}\)
C. \({\text{[Ar] 3}}{{\text{d}}^{{\text{10}}}}\)
D. \({\text{[Ar] 3}}{{\text{d}}^{\text{8}}}{\text{ 4}}{{\text{s}}^{\text{1}}}\)
What is the electron configuration of vanadium?
A. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{2}}}{\text{4}}{{\text{s}}^{\text{3}}}\)
B. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{3}}}{\text{4}}{{\text{s}}^{\text{2}}}\)
C. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{4}}}{\text{4}}{{\text{s}}^{\text{1}}}\)
D. \({\text{1}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{s}}^{\text{2}}}{\text{2}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{s}}^{\text{2}}}{\text{3}}{{\text{p}}^{\text{6}}}{\text{3}}{{\text{d}}^{\text{5}}}\)
What are the numbers of neutrons and electrons in the iodine ion, \(^{{\text{125}}}{{\text{I}}^ + }\)?
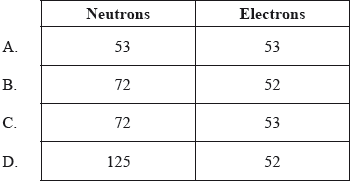
What is the abbreviated electron configuration of the telluride ion, \({\text{T}}{{\text{e}}^{2 - }}\)?
A. \({\text{[Kr]5}}{{\text{s}}^{\text{2}}}{\text{5}}{{\text{d}}^{{\text{10}}}}{\text{5}}{{\text{p}}^{\text{6}}}\)
B. \({\text{[Kr]5}}{{\text{s}}^{\text{2}}}{\text{4}}{{\text{d}}^{{\text{10}}}}{\text{5}}{{\text{p}}^{\text{2}}}\)
C. \({\text{[Kr]5}}{{\text{s}}^{\text{2}}}{\text{4}}{{\text{d}}^{{\text{10}}}}{\text{5}}{{\text{p}}^{\text{4}}}\)
D. \({\text{[Kr]5}}{{\text{s}}^{\text{2}}}{\text{4}}{{\text{d}}^{{\text{10}}}}{\text{5}}{{\text{p}}^{\text{6}}}\)
Consider the relative abundance of the isotopes of element X.
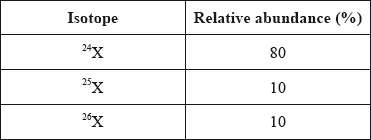
What is the relative atomic mass of X?
A. 24
B. 25
C. Between 24 and 25
D. Between 25 and 26
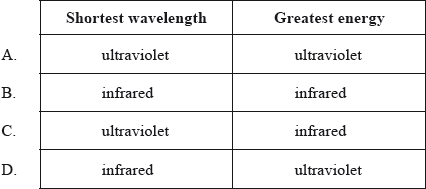
In the electromagnetic spectrum, which will have the shortest wavelength and the greatest energy?
Which shows the sub-levels in order of increasing energy in the fourth energy level of an atom?
A. \({\text{f}} < {\text{d}} < {\text{p}} < {\text{s}}\)
B. \({\text{p}} < {\text{d}} < {\text{f}} < {\text{s}}\)
C. \({\text{d}} < {\text{f}} < {\text{p}} < {\text{s}}\)
D. \({\text{s}} < {\text{p}} < {\text{d}} < {\text{f}}\)
What is the electron configuration of the \({\text{C}}{{\text{r}}^{2 + }}\) ion?
A. \({\text{[Ar]3}}{{\text{d}}^{\text{5}}}{\text{4}}{{\text{s}}^{\text{1}}}\)
B. \({\text{[Ar]3}}{{\text{d}}^{\text{3}}}{\text{4}}{{\text{s}}^{\text{1}}}\)
C. \({\text{[Ar]3}}{{\text{d}}^{\text{6}}}{\text{4}}{{\text{s}}^{\text{1}}}\)
D. \({\text{[Ar]3}}{{\text{d}}^{\text{4}}}{\text{4}}{{\text{s}}^{\text{0}}}\)
Which statement correctly describes the atomic emission spectrum of hydrogen?
A. It is a continuous spectrum converging at high frequency.
B. It is a line spectrum converging at high frequency.
C. It is a continuous spectrum converging at low frequency.
D. It is a line spectrum converging at low frequency.
Which electron configurations do not follow the Hund's rule?
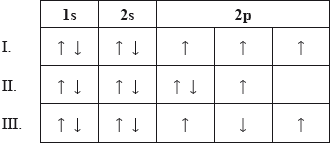
A. I and II only
B. I and III only
C. II and III only
D. I, II and III