
SL Paper 2
Lithium and boron are elements in period 2 of the periodic table. Lithium occurs in group 1 (the alkali metals) and boron occurs in group 3. Isotopes exist for both elements.
Every element has its own unique line emission spectrum.
(i) Define the terms atomic number, mass number and isotopes of an element.
Atomic number:
Mass number:
Isotopes of an element:
(ii) Distinguish between the terms group and period.
(iii) Deduce the electron arrangements of the lithium ion, \({\text{L}}{{\text{i}}^ + }\), and the boron atom, B.
\({\text{L}}{{\text{i}}^ + }\):
B:
(iv) Naturally occurring boron exists as two isotopes with mass numbers of 10 and 11. Calculate the percentage abundance of the lighter isotope, using this information and the relative atomic mass of boron in Table 5 of the Data Booklet.
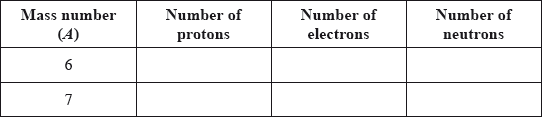
v) Lithium exists as two isotopes with mass numbers of 6 and 7. Deduce the number of protons, electrons and neutrons for each isotope.
(i) Distinguish between a continuous spectrum and a line spectrum.
(ii) Draw a diagram to show the electron transitions between energy levels in a hydrogen atom that are responsible for the two series of lines in the ultraviolet and visible regions of the spectrum. Label your diagram to show three transitions for each series.
(i) Explain why metals are good conductors of electricity and why they are malleable.
(ii) Iron is described as a transition metal. Identify the two most common ions of iron.
iii) Deduce the chemical formulas of lithium oxide and iron(II) oxide.
Lithium oxide:
Iron(II) oxide: