
SL Paper 1
What is the product of the reaction between hex-3-ene and steam?
A. Hexan-1-ol
B. Hexan-2-ol
C. Hexan-3-ol
D. Hexan-4-ol
Applying IUPAC rules, what is the name of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(C}}{{\text{H}}_{\text{3}}}{\text{)C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\)?
A. 2,3-dimethylpropanoic acid
B. Pentanoic acid
C. 3-methylbutanoic acid
D. 2-methylbutanoic acid
Which organic molecule is not a structural isomer of pentan-1-ol?
A. pentan-2-ol
B. 2-methylpentan-2-ol
C. 2-methylbutan-2-ol
D. pentan-3-ol
In which pair are both compounds secondary?
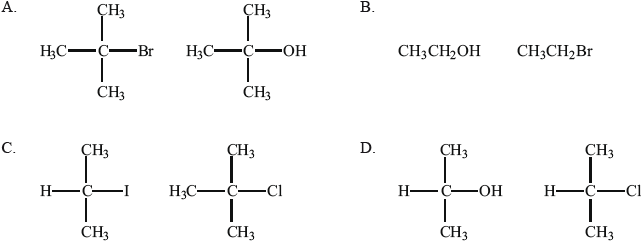
Which of the following pairs are members of the same homologous series?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\) and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\)
Which properties are features of a homologous series?
I. Same general formula
II. Similar chemical properties
III. Gradation in physical properties
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Some methane gas is burned in a limited supply of oxygen. Which products could form?
I. C(s)
II. CO(g)
III. CO2(g)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
How many structural isomers exist with the formula \({{\text{C}}_{\text{3}}}{{\text{H}}_{\text{5}}}{\text{C}}{{\text{l}}_{\text{3}}}\)?
A. 3
B. 4
C. 5
D. 6
What are possible products of the incomplete combustion of propane?
A. carbon monoxide, hydrogen and carbon
B. carbon dioxide, carbon and hydrogen
C. carbon, carbon monoxide and water
D. carbon dioxide and water only
Which equation represents a propagation step in the reaction of methane with bromine?
A. \({\text{C}}{{\text{H}}_{\text{4}}} \to {\text{C}}{{\text{H}}_{\text{3}}} \bullet + {\text{H}} \bullet \)
B. \({\text{C}}{{\text{H}}_{\text{4}}} + {\text{Br}} \bullet \to {\text{C}}{{\text{H}}_{\text{3}}} \bullet + {\text{HBr}}\)
C. \({\text{C}}{{\text{H}}_{\text{4}}} + {\text{Br}} \bullet \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{Br}} + {\text{H}} \bullet \)
D. \({\text{C}}{{\text{H}}_{\text{3}}} \bullet + {\text{Br}} \bullet \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{Br}}\)
Which species can oxidize ethanol to ethanoic acid?
A. \({{\text{I}}^ - }\)
B. Fe
C. \({{\text{O}}^{2 - }}\)
D. Acidified \({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}\)
What is the major product of the reaction between HCl and but-2-ene?
A. 1,2-dichlorobutane
B. 2,3-dichlorobutane
C. 1-chlorobutane
D. 2-chlorobutane
What is the mechanism for the reaction of propene with iodine in the dark?
A. electrophilic addition
B. electrophilic substitution
C. free radical substitution
D. nucleophilic substitution
What happens when a few drops of bromine water are added to excess hex-1-ene and the mixture is shaken?
I. The colour of the bromine water disappears.
II. The organic product formed does not contain any carbon-carbon double bonds.
III. 2-bromohexane is formed.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which substance can be polymerized to produce the polymer below?
A. But-1-ene
B. But-2-ene
C. Propene
D. 2-methylpropene
Which of the structures below is an aldehyde?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOC}}{{\text{H}}_{\text{3}}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH O}}\)
Which order is correct when the following substances are arranged in order of increasing boiling point?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\)
Which of the following are isomers of pentane?
I. 2-methylpentane
II. methylbutane
III. dimethylpropane
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which compound is not an isomer of hexane?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(C}}{{\text{H}}_{\text{3}}}{\text{)C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHCHC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
C. \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{3}}}{\text{CC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(C}}{{\text{H}}_{\text{3}}}{\text{)C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
Which steps are involved in the free-radical mechanism of the bromination of ethane in the presence of ultraviolet radiation?
I. \({{\text{C}}_2}{{\text{H}}_6} + {\text{Br}} \bullet \to {{\text{C}}_2}{{\text{H}}_5} \bullet + {\text{HBr}}\)
II. \({{\text{C}}_2}{{\text{H}}_5} \bullet {\text{B}}{{\text{r}}_2} \to {{\text{C}}_2}{{\text{H}}_5}{\text{Br}} + {\text{Br}} \bullet \)
III. \({{\text{C}}_2}{{\text{H}}_5} \bullet + {\text{Br}} \bullet \to {{\text{C}}_2}{{\text{H}}_5}{\text{Br}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which monomer could be used to form a polymer with the following repeating unit?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Cl}}\)
B. \({\text{C}}{{\text{H}}_{\text{2}}}{\text{ClC}}{{\text{H}}_{\text{2}}}{\text{Cl}}\)
C. \({\text{C}}{{\text{H}}_{\text{2}}}{\text{CHCl}}\)
D. CHClCHCl
Which organic product forms in the following reaction?
\[{{\text{(C}}{{\text{H}}_3}{\text{)}}_2}{\text{CHOH}}\xrightarrow[{{\text{reflux}}}]{{{{\text{K}}_2}{\text{C}}{{\text{r}}_2}{{\text{O}}_7}{\text{/}}{{\text{H}}^ + }}}\]
A. Ethanoic acid
B. Propanal
C. Propanone
D. Propanoic acid
Which compound contains a secondary carbon atom?
A. CH3CH(Cl)CH(CH3)2
B. (CH3)2CHCH2Cl
C. (CH3)3CCl
D. CH3CH2Cl
How many structural isomers of C6H14 exist?
A. 4
B. 5
C. 6
D. 7
Consider the compound \({\text{(C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{)CH=CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}\). Which statements are correct?
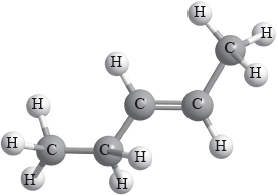
I. A suitable name is pent-2-ene.
II. The empirical formula is \({\text{C}}{{\text{H}}_{\text{2}}}\).
III. An isomer of the compound is pentane.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which of these reactions proceeds by a free radical mechanism in the presence of UV light?
A. C6H6 + Cl2 → C6H5Cl + HCl
B. C6H6 + 3H2 → C6H12
C. CH2CH2 + HBr → CH3CH2Br
D. CH3CH3 + Cl2 → CH3CH2Cl + HCl
Which is a tertiary halogenoalkane?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(C}}{{\text{H}}_{\text{3}}}{\text{)Cl}}\)
C. \({\text{C(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}{\text{Br}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHClC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
Which statement is correct about the polymerization of ethene to poly(ethene)?
A. The polymer is an alkene.
B. The monomer ethene and the repeating unit have the same empirical formula.
C. The monomer ethene is less reactive than the polymer.
D. The polymer contains C−C single and C=C double bonds.
Which structural formula represents a secondary halogenoalkane?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHBrC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
B. \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{3}}}{\text{CBr}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{3}}}{\text{Br}}\)
D. \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CHC}}{{\text{H}}_{\text{2}}}{\text{Br}}\)
What is the order of increasing boiling point?
A. C4H10 < CH3COOH < CH3CH2CHO < CH3CH2CH2OH
B. C4H10 < CH3CH2CHO < CH3CH2CH2OH < CH3COOH
C. CH3COOH < CH3CH2CH2OH< CH3CH2CHO < C4H10
D. C4H10 < CH3CH2CH2OH < CH3CH2CHO < CH3COOH
What is the organic product of the reaction between 2-chlorobutane and sodium hydroxide solution?
A. Butan-1-ol
B. Butan-2-ol
C. Butanal
D. Butanone
What is the name of the following molecule applying IUPAC rules?
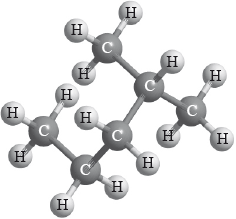
A. 1,1-dimethylbutane
B. Hexane
C. 2-methylpentane
D. 4-methylpentane
Which equation represents the initiation reaction when methane reacts with chlorine in the presence of ultraviolet light?
A. \({\text{C}}{{\text{H}}_{\text{4}}} \to {\text{C}}{{\text{H}}_{\text{3}}} \bullet + {\text{H}} \bullet \)
B. \({\text{C}}{{\text{l}}_2} \to {\text{2Cl}} \bullet \)
C. \({\text{C}}{{\text{l}}_2} \to {\text{C}}{{\text{l}}^ + } + {\text{C}}{{\text{l}}^ - }\)
D. \({\text{C}}{{\text{H}}_3} \bullet + {\text{C}}{{\text{l}}_2} \to {\text{C}}{{\text{H}}_3}{\text{Cl}} + {\text{Cl}} \bullet \)
What is the name of the compound with this molecular structure applying IUPAC rules?
A. 1-methylpropanoic acid
B. 2-methylpropanoic acid
C. 2-methylbutanoic acid
D. 3-methylbutanoic acid
Which statements about the chlorine free radical are correct?
I. It has 18 electrons.
II. It is an uncharged species.
III. It is formed by homolytic fission.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
What is the general formula of the alkyne series?
A. CnHn
B. CnH2n-2
C. CnH2n
D. CnH2n+2
Which compound can be oxidized when heated with an acidified solution of potassium dichromate(VI)?
A. CH3C(O)CH2CH3
B. CH3CH2CH(OH)CH3
C. (CH3)3COH
D. CH3(CH2)2COOH
Which conditions are used to convert ethanol to ethanal?
A. Excess oxidizing agent and reflux
B. Excess oxidizing agent and distillation
C. Excess ethanol and reflux
D. Excess ethanol and distillation
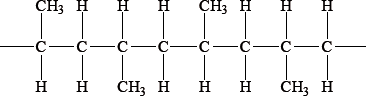
What is the name of the alkane shown in the diagram below, applying IUPAC rules?
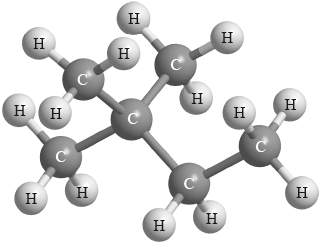
A. Hexane
B. 1,1,1-trimethylpropane
C. Ethylmethylpropane
D. 2,2-dimethylbutane
Which compound could be X in the two-stage reaction pathway?
\({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}} \to \) X \( \to {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}}\)
A. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\)
B. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{3}}}{\text{OH}}\)
C. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{Br}}\)
D. \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}\)
What is the product of the oxidation of butan-2-ol?
A. But-2-ene
B. Butanoic acid
C. Butanal
D. Butanone
What is the mechanism of the reaction between ethane and chlorine in sunlight?
A. Free radical substitutionC. Electrophilic substitution
D. Electrophilic addition
What is the name of \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{3}}}{\text{CCOC}}{{\text{H}}_{\text{3}}}\), applying IUPAC rules?
A. 2,2-dimethylbutan-3-one
B. 3,3-dimethylbutan-2-one
C. 2,2-dimethylbutanal
D. 3,3-dimethylbutanal
Which substance is not produced during the combustion of alkanes?
A. \({\text{C}}{{\text{O}}_{\text{2}}}\)
B. CO
C. C
D. \({{\text{H}}_{\text{2}}}\)
Which conditions are required to obtain a good yield of a carboxylic acid when ethanol is oxidized using potassium dichromate(VI), \({{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{7}}}{\text{(aq)}}\)?
I. Add sulfuric acid
II. Heat the reaction mixture under reflux
III. Distil the product as the oxidizing agent is added
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which are structural isomers?
I. CH3CH2OH and CH3OCH3
II. HOCH2CH3 and CH3CH2OH
III. CH3COOH and HCOOCH3
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
What is the IUPAC name for \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(C}}{{\text{H}}_{\text{3}}}{\text{)C}}{{\text{H}}_{\text{3}}}\)?
A. 1,1-dimethylpropane
B. 2-ethylpropane
C. 2-methylbutane
D. 3-methylbutane
Which statement about a homologous series is correct?
A. Members of the series differ by CH3.
B. Members of the series have the same physical properties.
C. Members of the series have the same empirical formula.
D. Members of the series have similar chemical properties.
Which three compounds can be considered to be a homologous series?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH, C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH, C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH, C}}{{\text{H}}_{\text{3}}}{\text{CHO, C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}{\text{, C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH, (C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}{\text{COH}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH, C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{, (C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\)
Which type of reaction occurs between an alcohol and a carboxylic acid?
A. Addition
B. Oxidation
C. Esterification
D. Polymerization
What is the IUPAC name of the following compound?
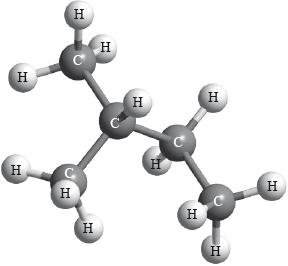
A. 2-methylbutane
B. Ethylpropane
C. 3-methylbutane
D. Pentane
Which equations represent the incomplete combustion of methane?
I. \({\text{C}}{{\text{H}}_4}{\text{(g)}} + {\text{2}}{{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\)
II. \({\text{C}}{{\text{H}}_4}{\text{(g)}} + {\text{1}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{CO(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\)
III. \({\text{C}}{{\text{H}}_4}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \to {\text{C(s)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
What product is formed when \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\) is reacted with acidified potassium dichromate(VI)?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOC}}{{\text{H}}_{\text{3}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
When bromine water is shaken with a liquid organic compound, it is rapidly decolourized. What can be determined from this test?
A. The compound is an alcohol.
B. The compound is an alkane.
C. The compound is an alkene.
D. The compound is an iodoalkane.
Which of the following statements about alkenes is not correct?
A. They have reactive double bonds.
B. They can form addition polymers.
C. They react mainly by substitution.
D. They can react with water to form alcohols.
Which compound would decolourize bromine water in the dark?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{4}}}{\text{OH}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHCHC}}{{\text{H}}_{\text{3}}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
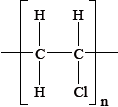
Which monomer is used to form the polymer with the following repeating unit?
A. CH3CH=CHCH3
B. CH3CH2CH=CH2
C. CH3CH2CH2CH3
D. (CH3)2C=CH2
Which compound could be formed when CH3CH2CH2OH is heated with acidified potassium dichromate(VI)?
I. CH3CH2CHO
II. CH3CH2COOH
III. CH3COCH3
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Applying IUPAC rules, what is the name of the compound?
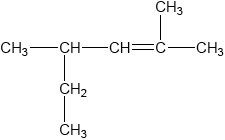
A. 1-ethyl-1,3-dimethylbut-2-ene
B. 2-ethyl-4-methylpent-3-ene
C. 2-methyl-4-ethylpent-3-ene
D. 2,4-dimethylhex-2-ene
Which compound is an isomer of octane, \({{\text{C}}_{\text{8}}}{{\text{H}}_{{\text{18}}}}\)?
A. \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CH(C}}{{\text{H}}_{\text{2}}}{{\text{)}}_{\text{2}}}{\text{CH(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\)
B. \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CHC}}{{\text{H}}_{\text{2}}}{\text{CHCHC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{5}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
D. \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CH(C}}{{\text{H}}_{\text{2}}}{{\text{)}}_{\text{2}}}{\text{CHCHC}}{{\text{H}}_{\text{3}}}\)
Which statement is correct for members of the same homologous series?
A. They have the same empirical formula and a gradual change in chemical properties.
B. They have the same empirical formula and a gradual change in physical properties.
C. They have the same general formula and a gradual change in chemical properties.
D. They have the same general formula and a gradual change in physical properties.
Which compounds belong to the same homologous series?
A. CHCCH2CH3, CHCCH2CH2CH3
B. CH3CH2CH2CH2OH, CH3CH2OCH2CH3
C. CH2CHCH3, CH3CH2CH2CH3
D. CH3COCH3, CH3CH2OCH3
Which molecule contains an ester group?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOC}}{{\text{H}}_{\text{3}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
D. \({\text{OHCC}}{{\text{H}}_{\text{2}}}{\text{CHO}}\)
Which three compounds can be considered to be a homologous series?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{N}}{{\text{H}}_{\text{2}}}\) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(N}}{{\text{H}}_{\text{2}}}{\text{)C}}{{\text{H}}_{\text{3}}}\) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{(NH)C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
C. \({\text{C(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{4}}}\) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\) \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CHC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\) \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOC}}{{\text{H}}_{\text{3}}}\) \({\text{HCOOC}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
Which type of reaction occurs when methanol and propanoic acid react together in the presence of a catalyst?
A. Addition
B. Condensation
C. Redox
D. Neutralization
What is the structural formula of 2,3-dibromo-3-methylhexane?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHBrCHBrCH(C}}{{\text{H}}_{\text{3}}}{\text{)C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHBrCBr(C}}{{\text{H}}_{\text{3}}}{\text{)C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHBrCBr(C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CHBrCHBrCH(C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\)
What is the function of the ultraviolet light used in the reaction between ethane and bromine?
A. It causes bromine free radicals to form bromine molecules.
B. It causes bromide ions to form bromine molecules.
C. It causes bromine molecules to form bromide ions.
D. It causes bromine molecules to form bromine free radicals.
The structure of a drug used to treat symptoms of Alzheimer’s disease is shown below. Which functional groups are present in this molecule?
A. Hydroxyl and ester
B. Hydroxide and ether
C. Hydroxyl and ether
D. Hydroxide and ester
Which functional group is present in paracetamol?
A. Carboxyl
B. Amino
C. Nitrile
D. Hydroxyl
Which alcohols are oxidized by acidified potassium dichromate(VI) solution when heated?
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
How many non-cyclic structural isomers exist with the molecular formula \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{10}}}}\)?
A. 2
B. 3
C. 4
D. 5
Which molecule has a tertiary nitrogen?
A. (CH3)2NH
B. (C2H5)4N+I−
C. C3H7N(CH3)2
D. C6H5NH2
What is the name of this compound, using IUPAC rules?
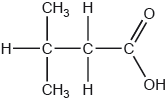
A. 1,1-dimethylpropanoic acid
B. 3,3-dimethylpropanoic acid
C. 2-methylbutanoic acid
D. 3-methylbutanoic acid
Which compound can both be esterified and turn acidified potassium dichromate(VI) solution green?
A. (CH3)3COHB. CH3CH2CO2H
C. (CH3)2CHOH
D. CH3CH2COCH3
Which of the following substances are structural isomers of each other?
I. \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
II. \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CHC}}{{\text{H}}_{\text{3}}}\)
III. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(C}}{{\text{H}}_{\text{3}}}{\text{)C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
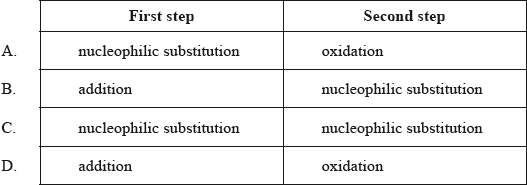
For the reaction pathway below, what are the names for the first and second steps?
\[{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHC}}{{\text{H}}_{\text{3}}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{CHClC}}{{\text{H}}_{\text{3}}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{CHOHC}}{{\text{H}}_{\text{3}}}\]
Which statements are correct for the reaction of ethene with bromine in the absence of ultraviolet light?
I. It is an addition reaction.
II. The organic product is colourless.
III. The organic product is saturated.
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
How many alcohols have the general formula C4H10O?
A. 3B. 4
C. 5
D. 6
From which monomer is this polymer made?
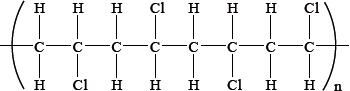
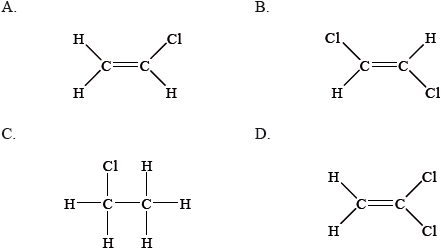
Which describes the reaction between a halogen and ethane?
What are possible names of a molecule with molecular formula C4H10O?
I. 1-Methoxypropane
II. 2-Methylpropan-2-ol
III. Butanal
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Which equation represents a propagation step in the mechanism for the reaction between ethane, \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{6}}}\), and chlorine, \({\text{C}}{{\text{l}}_{\text{2}}}\), in the presence of sunlight/UV?
A. \({{\text{C}}_2}{{\text{H}}_6} + {\text{Cl}} \bullet \to {{\text{C}}_2}{{\text{H}}_5} \bullet + {\text{HCl}}\)
B. \({{\text{C}}_2}{{\text{H}}_6} + {\text{Cl}} \bullet \to {{\text{C}}_2}{{\text{H}}_5}{\text{Cl}} + {\text{H}} \bullet \)
C. \({\text{C}}{{\text{l}}_{\text{2}}} \to 2{\text{Cl}} \bullet \)
D. \({{\text{C}}_2}{{\text{H}}_5} \bullet + {\text{Cl}} \bullet \to {{\text{C}}_2}{{\text{H}}_5}{\text{Cl}}\)
What is the name of the following compound applying IUPAC rules?
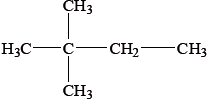
A. 1,1,1-trimethylpropane
B. 2,2-dimethylbutane
C. 3,3-dimethylbutane
D. 2-methyl-2-ethylpropane
What is the product of the following reaction?
\[{\text{C}}{{\text{H}}_3}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\xrightarrow{{{\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{2 - }/{{\text{H}}^ + }}}\]
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COOH}}\)
D. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\)
What are the functional groups in the aspirin molecule?
\(\begin{gathered} \begin{array}{*{20}{l}} {{\text{I.}}}&{{\text{Ether}}} \\ {{\text{II.}}}&{{\text{Carboxyl}}} \\ {{\text{III.}}}&{{\text{Ester}}} \end{array} \hfill \\ \hfill \\ \end{gathered} \)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
What is the name of this compound, using IUPAC rules?
A. 3-methylbutan-3-ol
B. 2-ethylpropan-2-ol
C. 2-methylbutan-2-ol
D. 3-methylbutan-2-ol