
SL Paper 3
Glucose, C6H12O6, is a monosaccharide that our body can use as a source of energy.
Deduce the equation for the cellular respiration of glucose.
Calculate the energy, in kJ, produced from 15.0g of glucose if its enthalpy of combustion is −2803kJmol−1.
Glucose is the basic building block of starch which can be used to make bioplastics. Outline two advantages and two disadvantages of biodegradable plastics.
Two advantages:
Two disadvantages:
Bioplastics are broken down by enzyme catalysed reactions. Sketch a graph illustrating how the rate of this reaction varies with pH.
Markscheme
C6H12O6 (aq) + 6O2 (aq) → 6CO2 (aq) + 6H2O (l)
Accept equations for anaerobic respiration, such as C6H12O6 (aq) → 2C3H6O3 (aq)
Ignore ATP if added as a product.
\(n\left( {{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}} \right)\left\langle { = \frac{{15.0}}{{180.18}}} \right\rangle = 0.0833 \ll {\rm{mol}} \gg \)
«energy=0.0833×2803=»233«kJ»
Award [2] for correct final answer.
Accept -233«kJ».
Two advantages:
renewable resource
broken down/digested by bacteria or other organisms within a relatively short time/quickly
reduce «volume of» plastic waste/landfill
reduce use of petrochemicals
OR
reduce use of fossil fuels as hydrocarbon source
degrade into non-toxic products
Any two advantages for [2 max].
M2: reference must be made to time. Do not accept “biodegradable” (since stated in question).
Ignore any mention of cost.
Two disadvantages:
require use of land «for crop production»
increased use of fertilizers/pesticides «leading to pollution»
OR
eutrophication
might break down before end of use
release of methane/CH4/greenhouse gas «during degradation»
Any two disadvantages for [2 max].
Ignore any mention of cost.
typical curve as shown in example above √
Accept any curve with a single maximum (not just bell-shaped).
Ignore features such as pH values on a pH scale or a pH value at maximum (if given).
Do not penalize if curve does not touch the x-axis.
Examiners report
Explain the solubility of vitamins A and C using section 35 of the data booklet.
Markscheme
Vitamin A:
fat soluble/soluble in non-polar solvents AND non-polar/long hydrocarbon backbone/chain
Vitamin C:
water soluble AND contains 4 hydroxyl groups/contains many hydroxyl groups/forms «many» H-bonds with water
Accept “Vitamin A: fat soluble/soluble in non-polar solvents as it contains only one hydroxyl group whose H-bonds with water are not strong enough to overcome London/dispersion/vdW forces between Vitamin A molecules”.
Accept “lipid” for “fats”.
Accept “alcohol” OR “hydroxy” OR “OH groups” for “hydroxyl” but not “hydroxide”.
Award [1 max] for “Vitamin A: fat soluble AND Vitamin C: water soluble” with no or incomplete explanation.
[2 marks]
Examiners report
Vitamins are micronutrients essential for good health.
Compare the solubilities of vitamins A and C in water by referring to the structures provided in Table 21 of the Data Booklet.
Describe the effect of deficiency of one of these vitamins and suggest two possible solutions.
Markscheme
vitamin A: not water-soluble because it has only one OH / is not very/less polar / contains long hydrocarbon group;
vitamin C: water-soluble because it has 4/many OH (and 1 C=O)/extensive hydrogen bonding;
Accept reference to polarity in one case but not in both.
effect: vitamin A: xerophthalmia/night blindness / vitamin C: scurvy / bleeding gums / less resistance to infection / bleeding lesions on legs/thighs / scorbutus;
Accept either of the following for the second mark:
solution for vitamin A: providing food composed of liver/fresh (orange and green) fruits/vegetables/spinach/eggs/carrots / providing genetically modified food containing vitamin A;
Examiners report
Most candidates answered that vitamin C is water soluble and vitamin A not, although some were vague in their explanations why.
Many candidates identified correctly the deficiency symptoms and named at least one solution.
A potato chip (crisp) was ignited and the flame was used to heat a test tube containing water.

(i) Calculate the heat required, in kJ, to raise the temperature of the water, using data in the table above and from Table 2 of the Data Booklet.
(ii) Determine the enthalpy of combustion of the potato chip, in \({\text{kJ}}\,{{\text{g}}^{ - 1}}\).
This energy comes mainly from the combustion of triglycerides. State the name of one other type of lipid found in the body and one role, other than energy storage, of this type of lipid.
Name:
Role:
Explain why lipids have a higher energy content than carbohydrates.
Markscheme
(i) \({\text{heat}} = \frac{{4.18 \times 20.0 \times (51.3 - 17.8)}}{{1000}}\);
\( = 2.80{\text{ (kJ)}}\);
(ii) \({\text{enthalpy of combustion}} = \left( {\frac{{2.80}}{{0.421}} = } \right){\text{ }} - 6.65{\text{ (kJ}}\,{{\text{g}}^{ - 1}}{\text{)}}\);
Name:
steroids;
Role:
(sex) hormones;
OR
Name:
phospholipids;
Role:
membranes;
lipids less oxidized/contain less oxygen / carbohydrates partially/more oxidized/contain more oxygen / OWTTE;
Examiners report
This part was generally well answered but there were some cases where 33.5 °C was converted into Kelvin. Many candidates had serious problems with unit conversions and gave the answer as 2800 J or 2800 kJ. Some candidates had correct value for (ii) but lost the mark because of the omission of the negative sign.
Part (b) was well answered.
Very few candidates linked the fact that lipids have higher energy content due to being less oxidized.
Lipids provide energy and are an important part of a balanced diet.
Identify the type of chemical reaction that occurs between fatty acids and glycerol to form lipids and the by-product of the reaction.
Arachidonic acid is a polyunsaturated omega-6 fatty acid found in peanut oil.
Determine the number of carbon–carbon double bonds present if the iodine number for the compound is 334. (Arachidonic acid Mr = 304.5)
Deduce the structure of the lipid formed by the reaction between lauric acid and glycerol (propane-1,2,3-triol) using section 34 of the data booklet.
Outline one impact food labelling has had on the consumption of foods containing different types of lipids.
Determine, to the correct number of significant figures, the energy produced by the respiration of 29.9 g of C5H10O5.
ΔHc (C5H10O5) = 205.9 kJ mol−1
Explain why lipids provide more energy than carbohydrates and proteins.
Markscheme
Type of reaction:
condensation
OR
esterification/triesterification
OR
nucleophilic substitution/nucleophilic displacement/SN2
By-product:
water/H2O
Do not accept just “substitution/displacement”.
[2 marks]
ALTERNATIVE 1
«\(\frac{{334}}{{253.8}}\) =» 1.32 AND «\(\frac{{100}}{{304.5}}\) =» 0.328
«\(\frac{{1.32}}{{0.328}}\) ≈» 4
ALTERNATIVE 2
«334 × \(\frac{{304.5}}{{100}}\) ≈» 1017
«\(\frac{{1017}}{{253.8}}\) ≈» 4
Award [2] for correct final answer.
[2 marks]
glycerol backbone
ester formula AND linkage
Accept a skeletal structure.
Penalize missing hydrogens or incorrect bond connectivities once only in Option B.
Accept condensed formula for ester.
[2 marks]
has affected consumption of trans-fats/cis-fats/saturated fats/unsaturated fats/hydrogenated/artificially altered fats
OR
reduce/eliminate trans-fats/increase in cis-fats
OR
reduce/eliminate saturated fats
OR
increase unsaturated fats
Do not accept “decrease in fat” alone.
Accept “lipid” for “fats”.
[1 mark]
«\(\frac{{29.9{\text{ g}}}}{{150.15{\text{ g mo}}{{\text{l}}^{ - 1}}}}\) =» 0.199 «mol»
«0.199 mol × 205.9 kJ mol–1 =» 41.0 «kJ»
Ignore significant figures in M1.
Award [2] for correct final answer.
Award [1 max] for incorrect significant figures in final answer.
[2 marks]
ratio of oxygen to carbon in lipids lower
OR
lipids less oxidized
OR
lipids more reduced
more energy per mass/g released when lipids are oxidized
Accept “«average» oxidation number of carbon in linoleic acid is lower” for M1.
[2 marks]
Examiners report
Polymers of α-glucose include the disaccharide maltose and the polysaccharide amylose, a type of starch. The cyclic structure of α-glucose is shown in section 34 of the data booklet.
State the specific type of linkage formed between α-glucose fragments in both maltose and amylose.
A person with diabetes suffering very low blood sugar (hypoglycaemia) may be advised to consume glucose immediately and then eat a small amount of starchy food such as a sandwich. Explain this advice in terms of the properties of glucose and starch.
Markscheme
«α-1,4-»glycosidic
Accept «α-1,4-»glycoside.
Accept “ether”.
[1 mark]
Glucose:
readily passes through intestine wall/dissolves in blood
OR
is immediately available for energy/respiration
OR
transported rapidly around body
Starch:
must be hydrolysed/broken down «into smaller molecules» first
[2 marks]
Examiners report
Anthocyanins are naturally occurring pigments responsible for the colour of blueberries and cranberries. The structures of two forms of anthocyanins are shown in Table 22 of the Data Booklet.
Using the abbreviations QB for quinoidal base and \({\text{F}}{{\text{C}}^ + }\) for flavylium cation, state an equation to describe how pH affects the colour of anthocyanins.
Suggest why blueberries should not be stored in aluminium cans.
Markscheme
\({\text{quinoidal base}} + {{\text{H}}^ + } \rightleftharpoons {\text{flavylium cation}}/{\text{QB}} + {{\text{H}}^ + } \rightleftharpoons {\text{F}}{{\text{C}}^ + }\);
Accept \( \to \) instead of \( \rightleftharpoons \)
blueberries are acidic and \({{\text{H}}^ + }\) ions react with aluminium to form \({\text{A}}{{\text{l}}^{3 + }}\);
\({\text{A}}{{\text{l}}^{3 + }}\) ions form (deeply) coloured (coordination) complexes with anthocyanins;
fruit discoloured;
\({\text{A}}{{\text{l}}^{3 + }}\) cause health problems / \({\text{A}}{{\text{l}}^{3 + }}\) deposited in bones instead of \({\text{C}}{{\text{a}}^{2 + }}\);
Examiners report
Most candidates correctly compared structural features of EGCG and rosmarinic acid in (a), but poorly demonstrated the application of knowledge of the factors that affect the colour of anthocyanins. One G2 respondent wondered whether candidates need to know the colours of pigments. This is clearly stated in F.4.3
Most candidates correctly compared structural features of EGCG and rosmarinic acid in (a), but poorly demonstrated the application of knowledge of the factors that affect the colour of anthocyanins. One G2 respondent wondered whether candidates need to know the colours of pigments. This is clearly stated in F.4.3
The straight chain form of glucose is represented below.
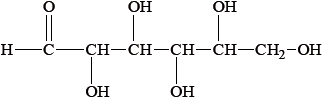
Fructose is an isomer of glucose, but they differ with regard to one functional group and hence in their redox properties.
Glucose is mainly present in one of two cyclic forms: \(\alpha \)-glucose and \(\beta \)-glucose. Distinguish between the two cyclic forms by completing the diagrams below.
(i) Identify the functional group present in glucose, but not fructose.
(ii) Identify the functional group present in fructose, but not glucose.
(iii) Identify the sugar that acts as a reducing agent.
Outline how the structure of cellulose is related to that of glucose.
Markscheme
\(\alpha \): C-1 OH below plane
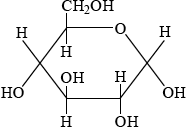 ;
;
\(\beta \): C-1 OH above plane
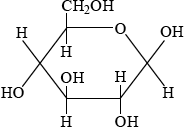 ;
;
(i) aldehyde/alkanal/CHO;
(ii) ketone/alkanone/CO;
(iii) glucose;
cellulose is (condensation) polymer of \(\beta \)-glucose;
(rings in cellulose) joined by \(\beta \)-1,4 linkages;
Examiners report
Part (a) was generally well answered with full marks awarded to more than half of the candidates. Those who did not score full marks usually reversed OH on carbon 4. Glucose has an aldehyde functional group which can undergo oxidation and this was covered in Topic 10. The fact that cellulose is a polymer of glucose and has beta-1,4 linkages seemed to have been overlooked by many candidates.
Glucose has an aldehyde functional group which can undergo oxidation and this was covered in Topic 10.
The fact that cellulose is a polymer of glucose and has beta-1,4 linkages seemed to have been overlooked by many candidates.
A mixture of the amino acids serine (Ser), glutamic acid (Glu) and lysine (Lys) was separated using electrophoresis and a buffer of pH 5.7. A drop containing the mixture was placed in the centre of the paper and a potential difference was applied. The amino acids were developed and the following results were obtained.
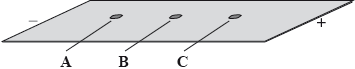
Describe how the amino acid spots may have been developed.
Predict which amino acid is present at spot C. Explain your answer.
The amino acid at spot B is at its isoelectric point. Describe one characteristic of an amino acid at its isoelectric point.
Explain, using equations, how the amino acid glycine (Gly) can act as a buffer
Markscheme
organic dye / ninhydrin;
glutamic acid/Glu;
isoelectric point is below pH of buffer / acts as an acid / loses \({{\text{H}}^ + }\);
becomes negatively charged;
balanced (positive and negative) charges / no overall charge / zwitterion;
amphoteric / buffer solution;
\({{\text{H}}_2}{\text{NC}}{{\text{H}}_2}{\text{COOH}} + {{\text{H}}^{\text{ + }}} \rightleftharpoons {{\text{H}}_3}{{\text{N}}^ + }{\text{C}}{{\text{H}}_2}{\text{COOH}}/{{\text{H}}_3}{{\text{N}}^ + }{\text{C}}{{\text{H}}_2}{\text{CO}}{{\text{O}}^ - } + {{\text{H}}^ + } \rightleftharpoons {{\text{H}}_3}{{\text{N}}^ + }{\text{C}}{{\text{H}}_2}{\text{COOH}}\);
\({{\text{H}}_2}{\text{NC}}{{\text{H}}_2}{\text{COOH}} + {\text{O}}{{\text{H}}^ - } \rightleftharpoons {{\text{H}}_2}{\text{NC}}{{\text{H}}_2}{\text{CO}}{{\text{O}}^ - } + {{\text{H}}_2}{\text{O}}/\)
\({{\text{H}}_3}{{\text{N}}^ + }{\text{C}}{{\text{H}}_2}{\text{CO}}{{\text{O}}^ - } + {\text{O}}{{\text{H}}^ - } \rightleftharpoons {{\text{H}}_2}{\text{NC}}{{\text{H}}_2}{\text{CO}}{{\text{O}}^ - } + {{\text{H}}_2}{\text{O}}\)
Accept \( \to \) instead of \( \rightleftharpoons \)
Examiners report
Many candidates described electrophoresis instead of stating that ninhydrin was used to develop the amino acid spots. The process of electrophoresis was detailed in the stem of the question, so candidates should have been able to determine what was required if the question had been read carefully.
Predicting which amino acid was closer to the positive electrode was challenging, although many candidates scored some marks for their reasoning.
The majority of candidates correctly described one characteristic of an amino acid at its isoelectric point.
In (b) very few could write equations to explain how glycine can act as a buffer. Most candidates answered in words only, even though equations were specifically requested. A G2 comment suggested that SL candidates did not need to know about buffers. This is clearly stated as a requirement in B.2.2.
Artificial food colourants have recently been linked to increased hyperactivity in children. Many foods are colourful because of the natural pigments they contain.
Explain why naturally-occurring pigments are coloured.
State the class of pigments that give carrots and tomatoes their colour.
Outline why this class of pigment is susceptible to oxidation, and the effect of oxidation on this pigment.
Markscheme
(ability to) reflect and absorb different wavelengths/frequencies/colours of visible light;
carotenoids;
Do not accept \(\beta \)–carotene.
presence of (multiple) carbon–carbon double bonds;
loss/bleaching of colour / loss of vitamin A activity / off odours;
Do not accept change of colour.
Examiners report
Part (a) was generally poorly answered. Many candidates found it difficult to explain why naturally occurring pigments are coloured in terms of their ability to absorb and reflect light.
Candidates commonly correctly stated anthocyanins as the pigments in cranberries and strawberries, but they mistakenly gave \(\beta \)-carotene or carotenes as the answer for (c) (i) instead of the name of the class of pigments, carotenoids.
Only the better candidates readily understood the connection of the carbon-carbon double bond to oxidation and its relationship to the colour of the pigment.
Rancidity is the perception of flavours in lipids that our senses perceive as off because of a disagreeable smell, taste, texture or appearance. The processes that create the off-flavours may be hydrolytic rancidity or oxidative rancidity in lipids.
Predict the products of hydrolytic rancidity of fats.
The hydrolysis of milk products is used in the making of cheese. State two conditions which increase the rate of hydrolysis of fats in milk.
Markscheme
(component) fatty acids;
glycerol/propane-1,2,3-triol/ \({\text{C}}{{\text{H}}_2}{\text{(OH)CH(OH)C}}{{\text{H}}_2}{\text{(OH)}}\);
(presence of) enzymes/lipase;
heat;
Examiners report
Some candidates scored well on this question, but there were many weak responses. Candidates needed to relate the packaging of potato crisps to the exclusion of oxygen and light in (a).
Some candidates scored well on this question, but there were many weak responses. Candidates needed to relate the packaging of potato crisps to the exclusion of oxygen and light in (a).
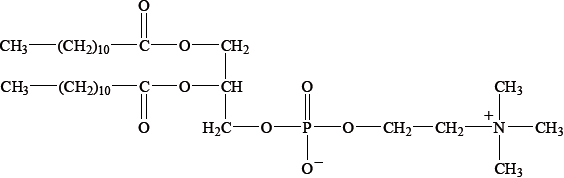
Many lipids are found in the human body. One type of lipid is a triglyceride.
Steroids and phospholipids are both classes of lipid found in the body. Cholesterol is a steroid. A structure of lecithin, a phospholipid, is shown below.
The formulas of some fatty acids are shown in Table 22 of the Data Booklet. State the equation for the reaction between glycerol and stearic acid to form a triglyceride.
Compare the structures of the two fatty acids: linoleic and linolenic acids.
State why these two fatty acids are so important in the human diet.
Distinguish between HDL and LDL cholesterol.
Compare the composition of cholesterol with a phospholipid such as lecithin.
Determine whether cholesterol or lecithin is more soluble in water.
Markscheme
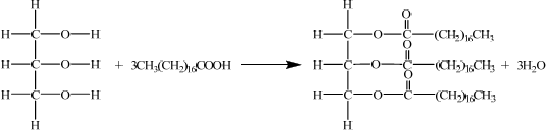
correct structure of glycerol and correct formula of stearic acid;
correct structure of triglyceride;
\({\text{3}}{{\text{H}}_{\text{2}}}{\text{O}}\) and coefficient of 3 on stearic acid;
Accept displayed or condensed formulas for molecules.
both have first double bond on C9 with carbon / linoleic has an \(\omega - 6{\text{ C=C}}\) double bond and linolenic acid has an \(\omega - 3{\text{ C=C}}\) double bond;
linoleic acid has 2 double carbon bonds and linolenic acid 3 double carbon bonds;
fatty acids are essential / body cannot synthesize them / OWTTE;
LDL is (a) larger (molecule) than HDL;
LDL transports cholesterol to arteries and HDL removes cholesterol from arteries;
LDL produced from saturated fats/trans fatty acids;
LDL increases the risk of heart disease/problems;
Accept converse statements for HDL.
Do not accept LDL is bad cholesterol and HDL is good cholesterol.
cholesterol is composed of C, H and O only and phospholipid contains C, H, O, P and N;
lecithin;
Examiners report
Candidates could not write an equation for the reaction between glycerol and stearic acid to form a triglyceride. Where candidates did write the correct equation they often did not balance the equation correctly.
In part (b) many candidates did not correctly recognize the difference in the number of carbon – carbon double bonds in the two fatty acids, nor the location of the double bonds and hence the significance of the omega-3 and omega-6 terminology.
Some candidates correctly identified that these fatty acids cannot be synthesised by the body and hence are essential.
In part (c) candidates could not distinguish between HDL and LDL, often referring simply and inadequately to ‘good’ and ‘bad’ cholesterol. Candidates had great difficulty comparing the composition of cholesterol with lecithin. An elemental comparison was required.
In part (c) candidates could not distinguish between HDL and LDL, often referring simply and inadequately to ‘good’ and ‘bad’ cholesterol. Candidates had great difficulty comparing the composition of cholesterol with lecithin. An elemental comparison was required.
In part (c) candidates could not distinguish between HDL and LDL, often referring simply and inadequately to ‘good’ and ‘bad’ cholesterol. Candidates had great difficulty comparing the composition of cholesterol with lecithin. An elemental comparison was required.
Saturated lipids found in butter and unsaturated lipids found in fish oil readily become rancid.
Identify the type of rancidity occurring in saturated lipids and the structural feature that causes it.
State one factor that increases the rate at which saturated lipids become rancid.
Butter contains varying proportions of oleic, myristic, palmitic and stearic acids. Explain in terms of their structures why stearic acid has a higher melting point than oleic acid, using section 34 of the data booklet.
Fish oil is an excellent dietary source of omega-3 fatty acids. Outline one impact on health of consuming omega-3 fatty acids.
Predict the solubility of retinol (vitamin A) in body fat, giving a reason. Use section 35 of the data booklet.
Explain why sharks and swordfish sometimes contain high concentrations of mercury and polychlorinated biphenyls (PCBs).
Plastics are another source of marine pollution. Outline one way in which plastics can be made more biodegradable.
Markscheme
hydrolytic «rancidity»
ester group
Accept a formula for ester group.
[2 marks]
«presence of» moisture/water
OR
«increase in» temperature
OR
«presence of» enzymes/bacteria/fungi/mould
OR
low pH/«presence of» acid
Accept “heat”.
[1 mark]
«stearic acid» straight chain/chain has no kinks/more regular structure
OR
«stearic acid» saturated/no «carbon–carbon» double bonds
«stearic acid» chains pack more closely together
stronger London/dispersion/instantaneous induced dipole-induced dipole forces «between molecules»
Accept “«stearic acid» greater surface area/electron density”.
[3 marks]
lowers risk of heart disease/atherosclerosis
OR
lowers LDL cholesterol
OR
increases HDL cholesterol
OR
aids brain/neurological development «in children»
OR
relieves rheumatoid arthritis
[1 mark]
soluble AND non-polar hydrocarbon chain
Accept as reasons “«predominantly» non-polar” OR “long hydrocarbon chain”.
[1 mark]
not biodegradable
OR
stored/accumulate in fat
biomagnification occurs
OR
concentration increases along food chain
Accept “stored/accumulate in bodies of prey/animals eaten”.
Accept “not excreted”.
[2 marks]
add starch/cellulose/carbohydrates/additives/catalysts «to plastic during manufacture to allow digestion by micro-organisms»
OR
replace traditional plastics with polylactic acid/PLA-based ones
OR
blend traditional and polylactic acid/PLA-based plastics
Accept reference to biodegradable plastics other than PLA; for example polyhydroxyalkanoates (PHA), poly(butylene succinate) (PBS), polybutylene adipate terephthalate (PBAT) and polycaprolactone (PCL).
[1 mark]
Examiners report
Vitamins can be water-soluble or fat-soluble.
Explain, at the molecular level, why vitamin D is soluble in fats. Use section 35 of the data booklet.
State one function of vitamin D in the body.
Markscheme
«mainly» hydrocarbon/non-polar «structure»
forms London/dispersion/instantaneous induced dipole-induced dipole forces «with fats»
Accept “forms van der Waals’/vdW forces”.
Award [1 max] for “contains only one OH/hydroxyl AND cannot form «enough» H-bonds”.
helps absorb calcium
OR
helps build bones
OR
helps keep bones healthy
OR
helps block the release of parathyroid hormone
OR
helps in muscle function
OR
helps immune system function
OR
cell growth
OR
reduction of inflammation
OR
protection from osteoporosis
OR
prevents rickets
Accept helps prevent colon/breast/prostate cancer.
Accept treat/prevent diabetes/heart disease/high blood pressure/multiple sclerosis.
Accept other correct answers.
Examiners report
Consider the following lipid and carbohydrate.
In order to determine the number of carbon-carbon double bonds in a molecule of linoleic acid, 1.24 g of the lipid were dissolved in 10.0 cm3 of non-polar solvent.
The solution was titrated with a 0.300 mol dm–3 solution of iodine, I2.
Determine the empirical formula of linoleic acid.
The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per gram than fructose.
State the type of reaction occurring during the titration.
Calculate the volume of iodine solution used to reach the end-point.
Outline the importance of linoleic acid for human health.
Markscheme
C9H16O
ratio of oxygen to carbon in linoleic acid lower
OR
linoleic acid less oxidized
OR
linoleic acid more reduced
Accept “«average» oxidation state of carbon in linoleic acid is lower”.
«electrophilic» addition/AE
OR
oxidation–reduction/redox
«\(\frac{{1.24\,{\text{g}}}}{{280.50\,{\text{g}}\,{\text{mo}}{{\text{l}}^{ - 1}}}}\) =» 0.00442 «mol»
0.00884 mol of C=C
OR
ratio of linoleic acid : iodine = 1:2
«volume of I2 solution = \(\frac{{0.00884\,{\text{mol}}}}{{0.300\,{\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}}}}\) =» 0.0295 «dm3» / 29.5 «cm3»
Award [3] for correct final answer.
Any two of:
increases «ratio of» HDL «to LDL» cholesterol
OR
decreases LDL cholesterol «level»
removes plaque from/unblocks arteries
OR
decreases risk of heart disease
decreases risk of stroke «in the brain»
Accept "essential fatty acid".
Do not accept “bad cholesterol” for “LDL cholesterol” OR “good cholesterol” for “HDL cholesterol”.
Do not accept general answers such as “source of energy” OR “forms triglycerides” OR “regulates permeability of cell membranes” etc.
[Max 2 Marks]
Examiners report
Green Chemistry reduces the production of hazardous materials and chemical waste.
Outline two specific examples or technological processes of how Green Chemistry has accomplished this environmental impact.
Markscheme
Any two of:
replaces plastics with biodegradable/starch/cellulose based plastics
use enzymes instead of polluting detergents/phosphates
OR
use of enzymes means lower temperatures can be used
OR
use enzymes instead of emulsifiers to treat oil spills
OR
use enzymes to produce esters at lower temperatures/without sulfuric acid
replace organic/toxic solvents with carbon dioxide
replace polymers from fossil fuel with bamboo/renewable resources
develop paint resins reducing production of volatile compounds «when paint is applied»
industrial synthesis of ethanoic/acetic acid from methanol and carbon monoxide has 100% atom economy
energy recovery
Accept formulas for names.
Award mark for any other reasonable specific green chemistry example that prevents the release of pollutants/toxic chemicals into the environment by changing the method or the materials used.
Do not award mark for methods that involve clean-up of pollutants from the environment such as host-guest chemistry or alternative energy sources.
[2 marks]
Examiners report
Food shelf life is the time it takes for a particular foodstuff to become unsuitable for eating because it no longer meets customer or regulatory expectations. As a result, in many parts of the world, packaged foods have a date before which they should be consumed.
Rancidity in lipids occurs by hydrolytic and oxidative processes.
State the meaning of the term rancidity as it applies to fats.
Compare the two rancidity processes.
Hydrolytic process:
Oxidative process:
Markscheme
unpleasant/disagreeable smell/taste/texture/appearance;
Hydrolytic process:
lipid converted into glycerol and fatty acid (by hydrolysis of water in presence of enzymes and no C=C present);
Oxidative process:
oxidation of unsaturated fatty acid (chains)/addition of oxygen across C=C/carbon-carbon double bond;
Examiners report
The vast majority of candidates answered correctly.
(b) (i) was poorly answered by the vast majority with only very few candidates scoring some marks here.
Amino acids are the building blocks of proteins.
Draw the dipeptide represented by the formula Ala-Gly using section 33 of the data booklet.
Deduce the number of 1H NMR signals produced by the zwitterion form of alanine.
Outline why amino acids have high melting points.
Markscheme
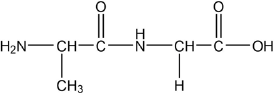
peptide bond
order of amino acids
Accept zwitterion form of dipeptide.
Accept a condensed structural formula or a skeletal structure.
Penalize missing hydrogens or incorrect bond connectivities once only in Option B.
[2 marks]
3
[1 mark]
form zwitterions
«strong» ionic bonding
OR
«strong» ionic lattice
OR
«strong» electrostatic attraction/forces
Do not accept hydrogen bonding or IMFs for M2.
[2 mark]
Examiners report
(a) Define the term genetically modified (GM) food.
(b) Discuss the benefits and concerns of using GM foods.
Markscheme
(a) food derived/produced from a GM organism;
(b) Benefits [3 max]:
crops:
enhanced taste/quality/appearance;
reduced maturation time;
increase in nutrients/yield;
improved resistance to disease/pests/herbicides;
enrichment of rice with vitamin A;
animals:
increased resistance;
increased productivity/feed efficiency;
better yield of milk/egg;
improved animal health;
environment:
“friendly” bio-herbicides/bio-insecticides;
conservation of soil/water/energy;
improved natural waste management;
Concerns [1 max]:
increased allergies;
risk of changing composition of balanced diet;
risk of GM genes (e.g. herbicide resistance) escaping to inappropriate areas of agriculture;
Examiners report
Many candidates were able to define the term genetically modified food well in (a). In (b) many of the candidates’ responses were very good, but there were cases with rather vague and journalistic answers.
Insulin was the first protein to be sequenced. It was determined that the end of one chain had the primary structure Phe–Val–Asn–Gln.
Paper chromatography can be used to identify the amino acids in insulin.
Draw the structural formula of a dipeptide containing the residues of valine, Val, and asparagine, Asn, using section 33 of the data booklet.
Deduce the strongest intermolecular forces that would occur between the following amino acid residues in a protein chain.
State the name of the process used to break down the insulin protein into its constituent amino acids.
Outline how the amino acids may be identified from a paper chromatogram.
Markscheme
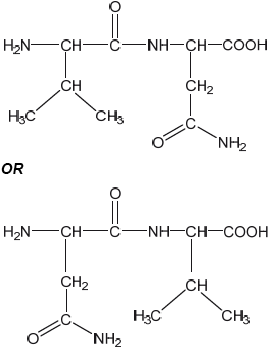
correct structures of Val AND Asn
correct amide link
[2 marks]
Phenylalanine and valine:
London/dispersion/instantaneous induced dipole-induced dipole forces
OR
permanent dipole-induced dipole «interactions»
Glutamine and asparagine:
hydrogen bonds
Do not accept dipole-dipole interactions.
[2 marks]
hydrolysis
[1 mark]
compare Rf with known amino acids
OR
compare distance moved with known amino acids
Accept “from Rf”.
[1 mark]
Examiners report
Enzyme activity depends on many factors. Explain how pH change causes loss of activity of an enzyme.
Markscheme
conformation/shape altered
OR
active site altered
OR
tertiary structure altered
acidic/basic/ionizable/COOH/carboxyl/NH2/amino groups in the R groups/side chains «react»
exchange/lose/gain protons/H+
ionic/H-bonds altered
Accept “substrate doesn't fit/fits poorly into active site” OR “enzyme denatures” for M1 but not “affects potential of
enzyme to form complex with substrate”.
Examiners report
Individual 2-amino acids have different structures depending on the pH of the solution they are dissolved in. The structures of serine and cysteine are given in Table 19 of the Data Booklet.
Deduce the structure of serine in
(i) a solution with a pH of 2.
(ii) a solution with a pH of 12.
Deduce the structure of serine at the isoelectric point.
Deduce the structures of the two different dipeptides that can be formed when one molecule of serine reacts with one molecule of cysteine.
Markscheme
(i) 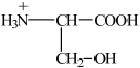 ;
;
If R– used or incorrect amino acid structure chosen from data book apply ECF for subsequent answers.
(ii) 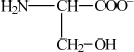 ;
;
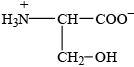 ;
;
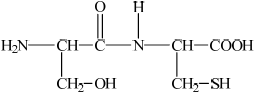 ;
;
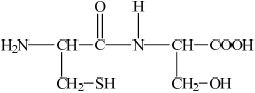 ;
;
Accept –CO–NH–/–CO–HN– for peptide linkage.
Examiners report
Most candidates simply drew the structure of the amino acid from the Data Booklet, and did not indicate the conjugate acid or base of the amino acid in solution in part (a).
Few knew how to draw the structure of the zwitterion in part (b). One G2 respondent commented that deducing the structure of an amino acid at varying pH levels is not on the syllabus. It is, in fact, referred to in B.2.2.
The better candidates were able to draw structures of two dipeptides.
Many weaker candidates were unable to create peptide links, and joined the molecules creatively but incorrectly.
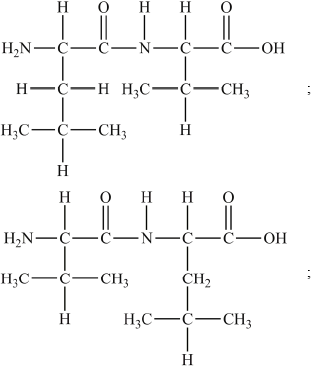
Proteins are natural polymers.
(a) List four major functions of proteins in the human body.
(b) Deduce the structures of two different tripeptides that can be formed when all three amino acids given below react together.

(c) Deduce the number of tripeptides that could be formed by using all three of the above amino acids to form a tripeptide.
(d) State the type of bonding that is responsible for the primary and secondary structures of proteins.
Primary:
Secondary:
(e) Describe and explain the tertiary structure of proteins. Include in your answer all the bonds and interactions responsible for the tertiary structure.
Markscheme
(a) structure / growth / repair
enzymes
hormones
transport
immunoproteins/antibodies
energy source
Two functions score [1].
(b) 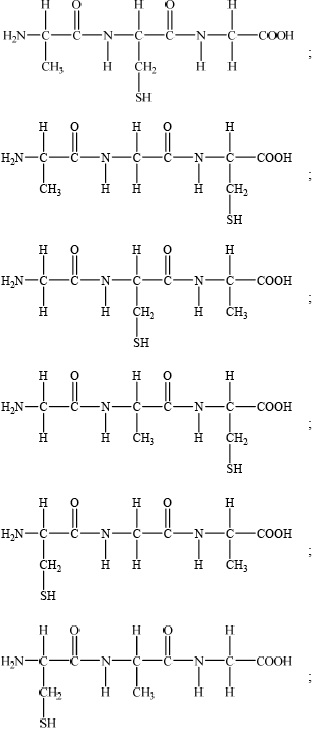
Accept CONH for peptide bond.
Penalize incorrect representation of peptide bond (e.g. COHN) once only.
(c) 6;
(d) 
(e) secondary structure folds to form a (unique) 3-D/dimensional structure of the protein;
Structure stabilized by:
covalent bonds / disulfide bridges
hydrogen/H-bonding
ionic bonds / salt bridges
van der Waals’/dispersion/London forces
Two bond types score [1].
Examiners report
Often quite well done. In part (a), whilst proteins can be used as an energy source, energy storage would not be considered a major function as the body usually stores energy in other forms. In Part (e), few candidates pointed out that the tertiary structure is a folding of the primary and secondary structures that gives the protein its three-dimensional shape.
Linoleic acid is an essential fatty acid whose formula is given in Table 22 of the Data Booklet. Determine the mass of iodine, I2, which reacts with 100 g of linoleic acid.
Fats, such as butter, are solid triglycerides. Explain why fats have a higher energy value than carbohydrates.
The formula of stearic acid is also given in Table 22 of the Data Booklet. Explain why linoleic acid has a lower melting point compared to stearic acid.
Markscheme
2 mol of iodine reacts with 1 mol of linoleic acid;
\({M_{\text{r}}} = {\text{253.80 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) for iodine and \({M_{\text{r}}} = {\text{280.50 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) for linoleic acid;
\(\left( {\frac{{(507.60 \times 100.00)}}{{280.50}} = } \right){\text{ }}180.96{\text{ (g)}}/181{\text{ (g)}}\);
Award [3] for correct final answer.
Allow 254 g mol–1 for iodine and 281/280 g\(\,\)mol–1 for linoleic acid.
Award [2 max] for incorrect ratio calculation to give answers such as 90.4, 90.481, 90.7 (g) depending on Mr values used.
less oxidized (compared to carbohydrates) / fewer oxygen atoms (compared to carbohydrates);
C=C’s in linoleic acid cause the chain to be more uneven/kinked;
linoleic acid cannot pack as closely as stearic acid;
intermolecular/van der Waal’s/London/dispersion forces weaker in linoleic acid;
Accept converse argument for stearic acid.
Examiners report
In (a) the vast majority of candidates did not recognise that linoleic acid had two C=C double bonds and hence the ratio n(I2):n(acid) = 2:1. Candidates also made careless errors in calculating the molar mass of either/both iodine or/and linoleic acid.
Part (b) was answered well by about half of the candidates.
In Part(c) many candidates scored both marks, but there were cases which indicated poor preparation of candidates on this rather trivial question which appears so often in examinations.
Proteins are vital components of living systems.
State the general formula of 2-amino acids.
State two characteristic properties of 2-amino acids.
Using Table 19 of the Data Booklet, deduce the structural formula of two dipeptides that could be formed by the reaction of alanine with serine and state the other product of the reaction.
Other product of the reaction:
Explain the difference between the primary and secondary structure of proteins.
State the predominant interaction responsible for the secondary structure.
Explain how a sample of a protein can be analysed by electrophoresis.
Markscheme
H2NCHRCOOH;
Allow various other combinations e.g. RCH(NH2)COOH etc. and allow NH2 and HOOC on right etc.
Allow structural formula if drawn, showing all the bonds.
Do not accept the formula of a specific amino acid.
isoelectric point;
formation of zwitterion/inner salt / \({{\text{H}}_{\text{3}}}{{\text{N}}^ + }{\text{CHRCO}}{{\text{O}}^ - }\);
(can act as a) buffer / has both acidic and basic properties / can react with \({{\text{H}}^ + }\) or \({\text{O}}{{\text{H}}^ - }\) / can exist as cations in acidic solution and anions in alkaline solution;
can form proteins/dipeptides/peptides / can react to form condensation products;
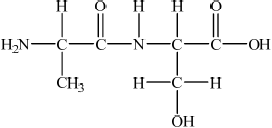 ;
;
 ;
;
Allow condensed structural formulas.
water/ H2O;
Primary structure:
(linear) sequence/order of amino acids / OWTTE;
Secondary structure:
way in which chain of amino acids folds itself / way in which sequence is kept together by hydrogen bonding between atoms in sequence / OWTTE;
Accept can exist as \(\alpha \)-helix or \(\beta \)-sheet.
hydrogen bonding/ H- bonding;
add hydrochloric acid/HCl / hydrolyse to convert protein into amino acid mixture / (successively) release amino acids;
mixture/amino acids spotted/placed on paper/gel;
Can be shown with diagram.
Do not accept protein placed/spotted on paper/gel.
use of buffer solution;
apply voltage/potential difference;
Can be shown with diagram.
Do not allow “pass current/electricity through mixture”.
amino acids move in different directions (depending on their isoelectric points);
develop with ninhydrin/triketohydrindane hydrate/2,2-dihydroxyindane-1,3-dione/organic dye;
measure distances moved / compare with known samples / measure isoelectric points (and compare with data);
Examiners report
Most attempts at (a) were successful.
A surprising number of candidates failed in (b) to give properties, as they misinterpreted the question and quoted structural features, such as the presence of an amino group.
Some students had difficulty deducing the structure of the two dipeptides in (c), however most candidates were able to identify the other product as water.
Most answers in (d) showed an understanding of protein primary and secondary structures.
Most answers in (d) showed an understanding of protein primary and secondary structures.
Responses to (e) were better than in some previous sessions; even so, full marks were rare, with many either omitting the hydrolysis of the protein or referring to a current being passed through the sample rather than a voltage being applied.
Proteins are macromolecules formed from 2-amino acids. Once a protein has been hydrolysed, chromatography and electrophoresis can be used to identify the amino acids present.
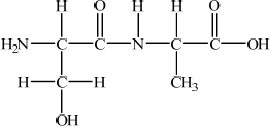
State the name of the linkage that is broken during the hydrolysis of a protein and draw its structure.
Explain how electrophoresis is used to analyse a protein.
Markscheme
peptide/amide;
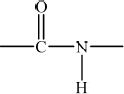 ;
;
Continuation bonds are needed for the mark.
add HCl/NaOH/enzyme (to hydrolyse the protein into amino acids);
mixture of amino acids is placed on the centre of a gel/PAGE/polyacrylamide/paper in buffer solution;
voltage/potential difference applied across gel;
Do not accept electric current.
different amino acids move to different distances according to their charge/isoelectric point / move at different rates towards oppositely charged electrodes;
gel/paper developed by spraying with ninhydrin/organic dye/can be detected by a stain/made to fluoresce under ultra-violet light;
distances moved/isoelectric points are compared with literature values;
Examiners report
It was surprising to see that quite a few candidates did not know the name of the linkage broken during the hydrolysis of a protein and only about half of the candidates, who stated the name could draw the structure of the peptide bond correctly. In some cases glycosidic or ester linkage appeared.
The explanation of how electrophoresis is used to analyse a protein was generally answered very well.
Low-density lipoproteins (LDL) can cause cholesterol to line the walls of the arteries and lead to cardiovascular disease. High-density lipoproteins (HDL) are smaller than low-density lipoproteins.
The formulas of linoleic acid and linolenic acid are given in Table 22 of the Data Booklet. Many vegetable oils are advertised as being a good source of omega-6 fatty acids whereas green leaves are a good source of omega-3 fatty acids.
(i) Identify the major source of low-density lipoproteins.
(ii) State the importance of high-density lipoproteins.
Compare the chemical structures of linoleic acid, an omega-6 fatty acid, and linolenic acid, an omega-3 fatty acid.
Markscheme
(i) saturated fats / fats derived from lauric/myristic/palmitic acid / the liver;
(ii) remove cholesterol from the arteries / transport cholesterol to the liver / protect against heart attack/disease;
both unsaturated fatty acids;
linoleic acid has one less C=C bond/carbon to carbon double bond/is less unsaturated / linolenic acid has one more C=C bond/carbon to carbon double bond/is more unsaturated;
in linoleic the first C=C bond occurs further from the end of hydrocarbon chain / in linolenic the first C=C bond occurs closer from the end of hydrocarbon chain;
both contain 18 carbon atoms;
Examiners report
Most candidates answered part (a) correctly. One respondent stated in the G2 form that ªstudents are not required to know a major source of LDL”, which is a fair comment and will be addressed in future paper editing.
Part (b)(i) proved more challenging and many candidates lost marks resulting from the use of vague terms as “double bonds” rather than “carbon to carbon double bond”. Weaker candidates merely copied the structures from the Data Booklet and even strong candidates often failed to correctly refer the position of the carbon to carbon double bonds.
Explain why raw meat changes colour from purplish-red to brown on standing.
Markscheme
purplish-red colour of meat is produced by myoglobin;
Accept heme.
Fe has oxidation state +2 in myoglobin;
(upon standing) oxidizes to \({\text{F}}{{\text{e}}^{3 + }}\) which is brown;
Examiners report
Many candidates had difficulties explaining the brown colouring of meat upon standing.
Many food items contain genetically modified ingredients.
Explain what is meant by the term genetically modified food.
Describe two advantages and one concern about the use of genetically modified food.
Markscheme
a food from an animal or plant in which the DNA/genetic material has been altered by artificial means / OWTTE;
advantages [2 max]:
quicker growth / reduced maturation time / more harvest per year;
increase resistance to disease/pests / less herbicides/pesticides / improved
plant/animal health;
more tolerant of climate/extending its range / lower water consumption;
increase in the yield/productivity/feed efficiency;
improve flavour;
incorporate beneficial substances;
increased shelf life;
Examiners report
Many students appeared unaware of the exact meaning of the phrase genetically modified. They were however better briefed on the advantages and possible concerns regarding foodstuffs from such sources.
Many students appeared unaware of the exact meaning of the phrase genetically modified. They were however better briefed on the advantages and possible concerns regarding foodstuffs from such sources.
Determine the number of double bonds in linoleic acid, \({{\text{C}}_{{\text{18}}}}{{\text{H}}_{{\text{32}}}}{{\text{O}}_{\text{2}}}\), and linolenic acid, \({{\text{C}}_{{\text{18}}}}{{\text{H}}_{{\text{30}}}}{{\text{O}}_{\text{2}}}\), and suggest which fatty acid will have a higher iodine number.
Explain why it is important to include the fatty acids linoleic and linolenic acid in a balanced diet.
The partial equation for the enzyme-catalysed hydrolysis of a triglyceride is shown below. Draw the structural formulas of the products A and B.
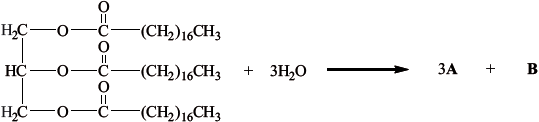
A:
B:
Deduce whether the fatty acid obtained in part (c) will have a higher or lower melting point compared to oleic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{7}}}{\text{CH=CH(C}}{{\text{H}}_{\text{2}}}{{\text{)}}_{\text{7}}}{\text{COOH}}\). Outline your reason.
Markscheme
linoleic has 2 C=C / double bonds and linolenic has 3 C=C / double bonds;
linolenic acid (will have higher iodine value);
Accept linoleic has 3 double bonds and linolenic has 4 double bonds.
essential fatty acids / cannot be synthesized in body;
lowers LDL cholesterol level / lowers risk of heart disease / affects inflammation / conversion to important molecules;
A: \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{{\text{16}}}}{\text{COOH}}\);
B: \({\text{C}}{{\text{H}}_{\text{2}}}{\text{OHCHOHC}}{{\text{H}}_{\text{2}}}{\text{OH}}\);
Accept [1 max] if A and B reversed.
Accept full structural formula.
Penalize missing H atoms once only.
higher (melting point);
saturated fatty acids / no unsaturation / no C=C bonds;
Accept appropriate reason such as close packing, no kink in molecule, stronger van der Waals’ forces, larger surface area of contact.
Accept opposite reasons why oleic acid would have a lower mp.
Examiners report
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.
Cholesterol belongs to a class of substances named lipids.
Identify the characteristic structural feature of cholesterol.
Identify two other types of lipids found in the human body.
State what the terms HDL and LDL represent.
Compare the structures of linoleic acid and linolenic acid.
Markscheme
steroid/steroidal backbone/4 ring/tetra cyclic carbon structure skeleton;
Do not accept OH, hydroxyl, hydroxide, alcohol.
Accept a correct sketch of the steroid backbone.
phospholipids;
triglycerides/triglycerols;
high density lipoprotein and low density lipoprotein;
both have 18 carbon atoms;
both have carboxyl/COOH;
linoleic acid has 2 double bonds and linolenic 3 / linoleic acid has less double bonds / linoleic acid is less unsaturated;
first double bond of linoleic is after the \({{\text{6}}^{{\text{th}}}}\) C atom and first of linolenic is after the \({{\text{3}}^{{\text{rd}}}}\) C atom from the end of the \({\text{C}}{{\text{H}}_{\text{3}}}\) group/counting from side of the chain that does not have COOH group / linoleic acid is omega-6 and linolenic acid is omega-3 / OWTTE;
Examiners report
Most candidates identified the steroid backbone.
Many candidates named only one other type of lipid.
Some candidates stated correctly the terms of HDL and LDL.
Most candidates compared at least two features of the structures of linoleic and linolenic acid.
Fats and vegetable oils are triesters of glycerol with fatty acids. Many of these acids contain 18 carbon atoms. The table shows the relative percentages of various \({{\text{C}}_{{\text{18}}}}\) fatty acid chains in four common fats and oils.
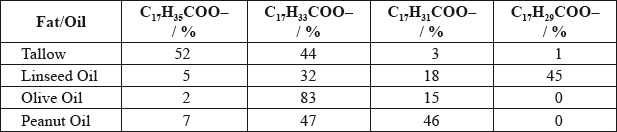
Deduce which fat or oil from the table could best be described as:
saturated
mono-unsaturated
poly-unsaturated.
Hydrogenation can result in the formation of trans fatty acids. Outline the meaning of the term trans fatty acids and explain why their formation is undesirable.
Markscheme
saturated: Tallow;
mono-unsaturated: Olive oil;
poly-unsaturated: Linseed oil;
3 correct award [2], 2 correct award [1], no marks for just one correct.
fats with trans configuration across the double bond;
not easily digested / accumulate in body tissue / increase LDL cholesterol levels;
Do not accept “bad cholesterol”.
Examiners report
Quite a few candidates could correctly identify the relative degree of saturation of the oils, though some thought they had to include all four in their answer. The meaning of shelf life was quite well known, in addition many realised that increasing unsaturation decreased shelf life and could suggest ways of increasing it. The conditions requiredcfor hydrogenation were not well appreciated, especially the need for a catalyst, and few could write any specific details regarding trans-fats apart from the fact there were health concerns regarding these.
Quite a few candidates could correctly identify the relative degree of saturation of the oils, though some thought they had to include all four in their answer. The meaning of shelf life was quite well known, in addition many realised that increasing unsaturation decreased shelf life and could suggest ways of increasing it. The conditions requiredcfor hydrogenation were not well appreciated, especially the need for a catalyst, and few could write any specific details regarding trans-fats apart from the fact there were health concerns regarding these.
Most foods are complex mixtures and many components of them are nutrients.
Identify the types of nutrients A, B and C.
A 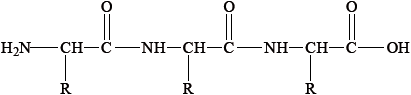
B 
C 
State the names of two types of nutrient other than those shown in part (b).
Markscheme
A: protein / polypeptide/tripeptide;
B: carbohydrate / sugar / monosaccharide / glucose;
C: lipid / triglyceride / vegetable oil / fat;
vitamins;
minerals;
water;
Examiners report
This question was generally well answered by many candidates. Though most had a general idea of the difference between a food and a nutrient, many did not appreciate the distinction between an “unhealthy” food and one that isn’t a nutrient.
This question was generally well answered by many candidates. Though most had a general idea of the difference between a food and a nutrient, many did not appreciate the distinction between an “unhealthy” food and one that isn’t a nutrient.
Petroleum (mineral oil) can be used either as a fuel or a chemical feedstock.
Name two fuels that are obtained from petroleum.
Describe one environmental problem that can result from the combustion of these fuels in the internal combustion engine and identify the specific combustion product responsible.
Plastic litter is an environmental problem that results from the use of petroleum as a chemical feedstock. Identify the property of plastics that is responsible for this.
One product that is made from crude oil is the chemical feedstock that can be used to synthesize commercial liquid-crystal displays. Discuss the properties that a substance must have to make it suitable for use as a liquid-crystal display.
Markscheme
Any two for [1]
petrol/gasoline
kerosene/paraffin/aviation fuel
diesel
fuel oil/gas oil
petroleum gas/refinery gas
global warming;
carbon dioxide;
OR
air pollution;
carbon monoxide / particulates / oxides of nitrogen/NO/\({\text{N}}{{\text{O}}_{\text{2}}}\) / \({\text{VO}}{{\text{C}}_{\text{s}}}\);
Accept oxides of sulphur/SO2.
OR
acid rain;
oxides of nitrogen/NO/\({\text{N}}{{\text{O}}_{\text{2}}}\);
Accept oxides of sulphur/SO2.
slow decomposition / not biodegradeable;
chemically stable;
liquid crystal phase over a suitable range of temperatures;
rapid switching speed;
Examiners report
In part (a) a significant number of candidates named two fuels obtained from petroleum.
A significant number of candidates described the environmental problem.
The non-biodegradable property of plastics was stated correctly by many candidates.
The properties of a material that made it suitable for use as a liquid crystal display demonstrated poor understanding by many candidates.
Foods derived from genetically modified organisms were introduced in the early 1990s. State one benefit and one concern of consuming genetically modified foods.
Benefit:
Concern:
Markscheme
Benefit:
enhanced taste/flavour/quality/nutrients/vitamin A / longer shelf life / greater yield / greater resistance to pesticides/diseases;
Concern:
increased allergies / changed composition of balanced diet / unknown health consequences in food chain / risk of escape to wild population / lack of knowledge of potential consequences to ecosystem;
Examiners report
The benefit and concern of consuming genetically modified foods was answered very well by the vast majority of candidates, but there were cases with rather vague and journalistic responses.
Starch and cellulose are polysaccharides found in many plants.
Compare the structures of starch and cellulose.
Markscheme
both are polymers of glucose / both contain glycosidic linkages;
starch is formed from \(\alpha \)-glucose / can have \(\alpha \)-1,6 linkages (and \(\alpha \)-1,4 linkages) / amylopectin form is branched;
cellulose is formed from \(\beta \)-glucose/has \(\beta \)-1,4 linkages / does not have 1,6 linkages / is not branched / is only straight-chain;
Examiners report
Many candidates had some idea of the α-glucose and β-glucose origins of starch and cellulose respectively, but it was disappointing to see weak answers where candidates compared other features/properties, such as digestibility or solubility, instead of comparing the structures as it was asked for.
Proteins are formed during condensation reactions of 2-amino acids.
Using Table 19 of the Data Booklet, deduce the structural formulas of the two dipeptides formed by the reaction of leucine (Leu) with valine (Val).
Dipeptide 1:
Dipeptide 2:
State the other substance formed during this reaction.
Explain how amino acids can be analysed using electrophoresis.
List two functions of proteins in the body.
Markscheme
Accept full or condensed structural formulas.
Penalize incorrect representation of peptide link (COHN or NHOC) once.
Award [1] for a correct peptide link if the rest of the structure is incorrect.
water/\({{\text{H}}_{\text{2}}}{\text{O}}\);
sample of amino acids/mixture placed/spotted on gel/polyacrylamide/PAGE/paper and buffer solution/solution of known pH;
potential difference/voltage applied;
Do not accept current.
Allow potential for potential difference.
Allow electric field applied.
if the (amino acid’s) isoelectric point is below the pH (of buffer) it is negatively charged / if the (amino acid’s) isoelectric point is above the pH (of buffer) it is positively charged;
different amino acids move different distances/rates according to their charge/isoelectric point / different amino acids move at different rates towards oppositely charged electrodes / OWTTE;
spray/develop with ninhydrin/organic dye / detected by staining/fluorescence under UV light;
measure distance travelled and compare with standards/isoelectric points;
Any two for [1]
structural / growth / repair
Allow more specific function eg, forms tendons/muscles/eye lens/ nails/hair, repair of tissue/cells etc.
enzyme / biological catalyst
hormone / chemical messenger
transport of molecules
Allow movement/carriage of molecules, and chemicals instead of molecules / OWTTE.
Do not award mark for transport alone.
storage of molecules
Do not award mark for storage alone.
Allow chemicals instead of molecules / OWTTE.
lubrication
(to make/produce) immunoproteins/antibodies
energy source
Do not accept energy storage.
Allow more specific examples of any of the above.
Examiners report
Part (a) was generally well answered. There were instances of careless mistakes where the side-chain of the amino acid was incorrectly copied or connected, and some instances where peptide links were incorrectly represented as COHN resulting in the loss of one mark. However, there were many cases where candidates had totally incorrect links between the amino acids in the dipeptide, and some scripts did not even attempt to connect the two amino acids.
Part (a) was generally well answered. There were instances of careless mistakes where the side-chain of the amino acid was incorrectly copied or connected, and some instances where peptide links were incorrectly represented as COHN resulting in the loss of one mark. However, there were many cases where candidates had totally incorrect links between the amino acids in the dipeptide, and some scripts did not even attempt to connect the two amino acids.
In part (b), all candidates were reasonably familiar with describing electrophoresis and some of them tried to explain how electrophoresis separated amino acids, but most candidates did not show a thorough understanding of the technique and only scored partial marks out of the 4 marks allocated. Most candidates did not clarify that amino acids moved at different rates through the gel depending on their charge (at the buffer’s pH), or the relation between isoelectric point, buffer pH and charge on amino acid.
The majority of candidates stated two correct functions of proteins in part (c). Energy storage was not accepted, and transport needed mention of molecules.
Naturally occurring pigments give many foods their distinctive colours.
Chlorophyll is a pigment found in green vegetables.
A student decided to investigate the effect of sodium hydrogencarbonate, \({\text{NaHC}}{{\text{O}}_{\text{3}}}\), and vinegar on the colour of cooked green peas. Her results are shown below:
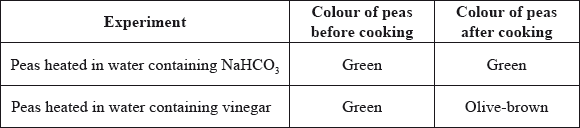
List two factors which may affect the colour stability of a pigment.
State how the sodium hydrogencarbonate maintains the green colour of the peas.
The structure of chlorophyll is shown in Table 22 of the Data Booklet. Describe what happens to the structure of chlorophyll when the peas are heated in water containing vinegar.
Markscheme
temperature (changes);
light;
pH (change);
presence of metal ions;
oxidation/reduction;
\({\text{HCO}}_3^ - \) produces a (slightly) alkaline/basic pH / pH range 7.5–9 / OWTTE;
Accept “buffers the solution”.
Mg2+/magnesium (ion) displaced by (two) H+/hydrogen (ions);
Accept Mg2+/magnesium (ion) is released.
Examiners report
This question was very poorly answered. Whilst many candidates correctly identified factors affecting the stability of pigments, hardly any displayed the detailed knowledge required regarding the degradation of chlorophyll and the way that sodium hydrogencarbonate and vinegar affect the process. The Maillard reaction was even less well understood with only a handful of candidates gaining any marks.
This question was very poorly answered. Whilst many candidates correctly identified factors affecting the stability of pigments, hardly any displayed the detailed knowledge required regarding the degradation of chlorophyll and the way that sodium hydrogencarbonate and vinegar affect the process. The Maillard reaction was even less well understood with only a handful of candidates gaining any marks.
This question was very poorly answered. Whilst many candidates correctly identified factors affecting the stability of pigments, hardly any displayed the detailed knowledge required regarding the degradation of chlorophyll and the way that sodium hydrogencarbonate and vinegar affect the process. The Maillard reaction was even less well understood with only a handful of candidates gaining any marks.
Stearic acid, oleic acid and linolenic acid are all fatty acids that contain 18 carbon atoms. Their structures are given in Table 22 of the Data Booklet.
Partial hydrogenation of linolenic acid may lead to a product known as a trans fatty acid.
Explain which acid has the highest melting point.
Discuss two potential problems or health concerns associated with trans fatty acids.
Markscheme
stearic acid;
saturated molecule / more closely packed / greater surface area (of contact) / not “kinked”;
more/stronger van der Waals’ forces;
Accept intermolecular/London/dispersion forces instead of van der Waals’ forces.
trans fats harder to metabolize / accumulate in tissue;
increase levels of LDL cholesterol/low-density lipoprotein / increase risk of heart disease;
low-quality energy source;
Examiners report
Even though the correct acid was often not identified, explanations for the highest melting point often gained full credit in (a). Disappointingly few candidates were able to write a correct equation in (b) (with the addition of three moles of \({{\text{H}}_{\text{2}}}\) (g)) although the conditions were usually correct. In (c), many were able to show a trans orientation – but didn’t use a fatty acid – and many did not score both marks in (ii).
Even though the correct acid was often not identified, explanations for the highest melting point often gained full credit in (a). Disappointingly few candidates were able to write a correct equation in (b) (with the addition of three moles of \({{\text{H}}_{\text{2}}}\) (g)) although the conditions were usually correct. In (c), many were able to show a trans orientation – but didn’t use a fatty acid – and many did not score both marks in (ii).
Fats and oils have some similarities and some differences in their chemical structures.
State two major differences in their structures.
Describe how an oil can be converted into a fat.
Discuss two advantages and two disadvantages of converting oils into fats.
Markscheme
oils contain at least one C=C/carbon to carbon double bond;
oils have fewer carbon atoms in the hydrocarbon chains / OWTTE;
hydrogenation / react with hydrogen (gas);
heat/140−225 °C and metal catalyst/Ni/Zn/Cu/pressure;
Advantages: [2 max]
increases melting points / changes oil to a semi-solid/solid;
decreases rate of oxidation;
increases hardness;
controls feel/plasticity/stiffness;
Disadvantages: [2 max]
the more saturated the less good for the heart / OWTTE;
trans-fatty acids can be formed (through partial hydrogenation);
trans-fatty acids are difficult to metabolize / increase LDL levels / low quality energy source / accumulate in fatty tissue / are difficult to digest/excrete (from the body);
Examiners report
Most candidates compared structural features of fats and oils, but many failed to score as they missed the required specificity of carbon to carbon double bond in (a). A significant number of candidates compared melting points which was not part of the question and very few were able to state the difference in the length of hydrocarbon chains.
Many candidates gave detailed descriptions of the process to score both marks in part (b), but some failed to score the second mark by omitting the need of a catalyst/pressure and/or heat.
Many candidates were able to correctly suggest two advantages but failed to correctly state two disadvantages in part (c). Very often marks were lost as result of poor use of subject specific terms.
Suggest, in terms of its structure, why vitamin D is fat-soluble using section 35 of the data booklet.
Markscheme
«mostly» non-polar
OR
hydrocarbon backbone
OR
only 1 hydroxyl «group so mostly non-polar»
Accept “alcohol/hydroxy” for “hydroxyl” but not “hydroxide”.
[1 mark]
Examiners report
Simple sugars are nutrients and are also described as monosaccharides.
State three characteristic features of all monosaccharide molecules.
Markscheme
they have the empirical formula CH2O;
they contain one carbonyl/C=O group;
they contain at least two hydroxy/–OH groups;
Examiners report
Many candidates stated physical properties of monosaccharides rather than structural features. Those who stated structural features often only gave one or two rather than the three required.
The structures of retinol (vitamin A) and vitamin D are given in Table 21 of the Data Booklet. Deduce whether each vitamin is water-soluble or fat-soluble and explain your answer by referring to their structures.
Markscheme
both fat soluble;
both contain mainly non-polar/hydrocarbon parts (and only one OH group) / OWTTE;
Do not award ECF if water soluble is stated for either vitamin.
Do not award M2 for answers such as “since both do not have many OH groups present”.
Examiners report
The majority of candidates identified the solubility of both vitamins correctly in part (a). The explanation was correctly scored by only half of the candidates, while the rest talked about the OH group and did not discuss the main part of the molecule.
Starch and cellulose are polysaccharides found in plants.
Compare the structural features of starch and cellulose.
Humans can digest starch but cannot digest cellulose. Explain why humans cannot digest cellulose.
Markscheme
both are polymers of glucose;
starch has \(\alpha \)-1,4 (and \(\alpha \)-1,6) linkages / bonds / \(\alpha \) glucose;
cellulose has \(\beta \)-1,4 linkages / bonds / \(\beta \) glucose;
absence of cellulase enzyme (in humans);
Examiners report
Very few candidates seemed familiar with the structures of the required polysaccharides and whilst most knew that cellulose could not be digested because humans lack the required enzyme, far fewer could name this enzyme.
Very few candidates seemed familiar with the structures of the required polysaccharides and whilst most knew that cellulose could not be digested because humans lack the required enzyme, far fewer could name this enzyme.
Lactose is a disaccharide formed by the condensation reaction of the monosaccharides galactose and glucose.
Describe what is meant by a condensation reaction.
Draw the structure of galactose on the skeleton provided.
Explain how the inclusion of carbohydrates in plastics makes them biodegradable.
Markscheme
«reaction in which» two reactants/molecules/functional groups bond/react «to form a larger molecule/single main product»
small/tiny molecule
OR
H2O formed
Accept formula or name of a specified small molecule other than water such as ammonia, ethanoic/acetic acid,
ethanol, hydrogen sulfide etc. for M2.
Do not accept just “molecule formed”.
Award [1 max] for an example giving an equation of a condensation reaction such as the formation of a disaccharide.
Accept “alpha” or “beta” form of galactose.
Any two of:
makes the plastic more hydrophilic/water soluble
carbohydrates are broken down/hydrolysed by bacteria/microorganisms
makes plastic more accessible to bacteria as holes/channels are created
OR
plastic of lower density is more permeable/susceptible to water/oxygen/heat/pressure
weakens intermolecular/London/dispersion/instantaneous induced dipole-induced dipole forces «between polymer chains in the plastic»
Accept “van der Waals/vdW” for “London” forces.
[Max 2 Marks]
Examiners report
Triglycerides are one of three types of lipid found in the human body. The following equation represents the formation of a triglyceride.
X + 3RCOOH \( \rightleftharpoons \) triglyceride + 3Y
Identify the compounds X and Y.
X:
Y:
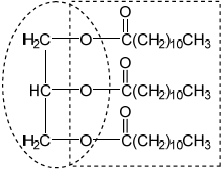
Draw the structural formula of a triglyceride formed from one molecule each of octanoic acid, lauric acid and stearic acid. The formulas of the acids are shown in Table 22 of the Data Booklet.
Explain whether the triglyceride in part (b) is a solid or a liquid at room temperature.
Identify the type of reaction that occurs during the formation of a triglyceride.
Explain why fats have a higher energy value per mole than carbohydrates.
Markscheme
X is glycerol/propane-1,2,3-triol/ \({\text{C}}{{\text{H}}_2}{\text{(OH)CH(OH)C}}{{\text{H}}_2}{\text{(OH)}}\);
Y is water/ \({{\text{H}}_2}{\text{O}}\);
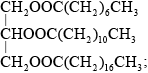
Accept the fatty acids in any order.
solid as contains (three) saturated/straight fatty acid chains;
can pack closer together;
have stronger London/dispersion/van der Waals’ forces between chains;
esterification / condensation;
fats contain less oxygen than carbohydrates / are in a less oxidised state (so more energy is released);
Examiners report
This question which was expected to be fairly straightforward proved to be rather tricky for candidates. In part (a) very few correctly identified glycerol in the formation of a triglyceride.
Drawing the structure of a triglyceride in (b) was challenging for many.
Some candidates explained very well why the triglyceride was a solid at room temperature, but others could only state that it was solid and were unclear of the reasons.
Only the best candidates could explain why fats have a higher energy value per mole than carbohydrates.
Foods such as pasta are rich in carbohydrates.
Monosaccharides are a type of carbohydrate.
State why a professional cyclist would eat pasta before a race.
(i) Fructose, a monosaccharide, is found in honey. Draw the straight-chain structure of fructose.
(ii) Draw the five-membered ring structure of \(\beta \)-fructose.
Markscheme
(source of) energy;
(i) 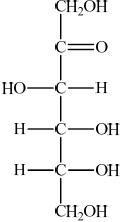 ;
;
Accept any six-carbon linear structure in which the second carbon is a carbonyl and there is one OH on all other carbons.
(ii) 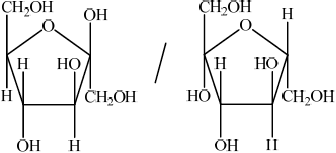 ;
;
Correct orientation of groups is required.
Allow Haworth projection, ie,
Examiners report
Almost all candidates related pasta to energy.
(i) Only a few candidates were able to draw the straight-chain structure of fructose.
(ii) About a quarter of the candidates were able to draw the five-membered ring structure of \(\beta \)-fructose. Many candidates had the orientation that suggested they drew the structure by referring to the structure of sucrose in the Data Booklet. A common mistake was missing the –OH at the position of the glycosidic link in sucrose.
Most foods contain nutrients.
Triglycerides are formed by the reaction of propane-1,2,3-triol (glycerol) with fatty acids.
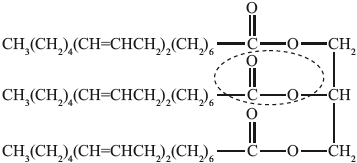
(i) State the name of the functional group circled in the triglyceride.
(ii) Identify the other product of the reaction.
(i) State the difference in structure between the fatty acids found in an oil and those in a fat.
(ii) Comment on the relative stability of oils and fats and state the names of two possible types of degradation reaction.
Markscheme
(i) ester;
(ii) water/\({{\text{H}}_{\text{2}}}{\text{O}}\);
(i) (fatty acids in) oils are unsaturated/contain (many) C=C/carbon-carbon double bonds / (fatty acids in) fats are (mostly) saturated/contain no/few/fewer (than oils) C=C /carbon-carbon double bonds;
(ii) C=C bonds degrade/oxidize more rapidly / oils become rancid more rapidly / fats are more stable;
Award [1 max] for any two of:
auto-oxidation;
Allow oxidative rancidity.
Do not accept “reaction with oxygen” (name required).
photo-oxidation;
Do not accept light.
microbial rancidity;
hydrolysis;
Allow hydrolytic rancidity.
Do not accept “addition of water” (name required).
Do not accept hydrogenation (since not a degradation reaction).
Examiners report
In (b) (i), the better candidates stated ester. The weaker candidates incorrectly suggested either alcohol or carboxylic acid. Water was universally known in (ii).
(c) was well answered though some did not score full marks by suggesting that hydrogenation is a degradation reaction which is incorrect.
Fats are complex molecules derived from fatty acids and glycerol. They are an important part of our diet and have many functions in the body including energy storage.
Identify the main functional group present in
(i) all fats.
(ii) all fatty acids.
Markscheme
(i) ester;
(ii) carboxylic acid / carboxyl;
Accept alkanoic acid.
Accept formulas.
Examiners report
In (a) the ester in fats was less well known than the carboxylic acid in fatty acids.
Food chemistry and nutritional science are two important scientific fields to which the general public relate.
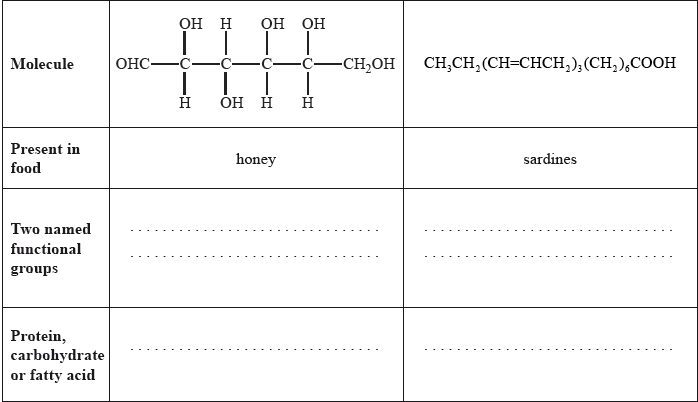
State two named functional groups present in each of the following molecules found in two different food products (honey and sardines). Identify each molecule as a protein, a carbohydrate or a fatty acid.
Butter is an example of a saturated fat and olive oil is an example of an unsaturated fat. Describe the main structural difference between these two types of fat.
Linoleic acid, whose structure is given in Table 22 of the Data Booklet, is present in peanut oil. The oil can be converted to a semi-solid using hydrogen gas. Predict the structural formula of the compound formed from the partial hydrogenation reaction of linoleic acid, and state a suitable catalyst for this reaction.
Structural formula:
Catalyst:
Partial hydrogenation can sometimes produce trans fats. Suggest why trans fats are considered unhealthy.
Olestra, with one of its structures shown below, has been used to prepare snacks such as crisps (potato chips). Deduce the type of compound that can undergo an esterification reaction involving carboxylic acid to produce olestra.
Olestra
Markscheme
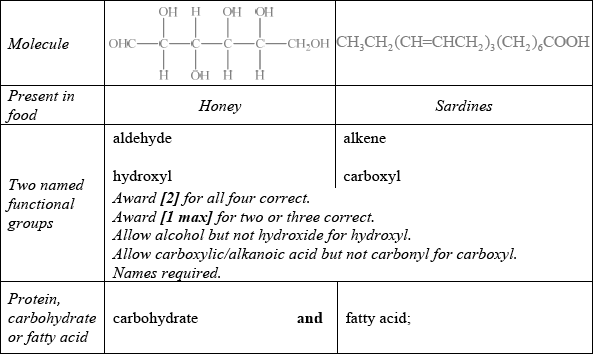
Saturated fat: no carbon-carbon double bonds/no C=C/all single carbon-carbon bonds/ all C–C and Unsaturated fat: carbon-carbon double bonds/C=C/alkene groups;
Mention of carbon-carbon or alkene necessary for mark.
Structural formula:
\({\text{C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_4}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{CH}}\)=\({\text{CH(C}}{{\text{H}}_2}{{\text{)}}_7}{\text{COOH}}\) /
\({\text{C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_4}{\text{CH}}\)=\({\text{CHC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{{\text{(C}}{{\text{H}}_2}{\text{)}}_6}{\text{COOH}}\);
Catalyst: nickel/Ni / palladium/Pd / platinum/Pt / copper/Cu / zinc/Zn;
decrease (blood) levels of HDL/high-density lipoprotein cholesterol (which protects from heart disease) / increase levels of LDL/low-density lipoprotein cholesterol (increasing risk of heart disease) / less easily digested/metabolized / leads to blocked arteries;
carbohydrate / disaccharide;
Allow sucrose / sugar.
Examiners report
Well answered with most candidates gaining two marks. The most common mistake was failing to recognize OHC– as an aldehyde.
The majority of candidates distinguished between saturated and unsaturated fats correctly.
The catalyst was usually well suggested but the structural formula was only provided by the strongest candidates. Some candidates gave the saturated product not taking note of the word “partial” in the question.
Another question better answered than in previous sessions. Many candidates related trans fats to LDL cholesterol.
This was a discriminating question. Only a few candidates recognized the compound as a carbohydrate.
Papain is a globular protein which is present in papaya fruit. Part of the sequence of its polypeptide chain is Gly–Cys–Val–Gly.
In the analysis of proteins, mixtures of amino acids with different isoelectric points can be separated using electrophoresis.
Proteins such as papain are formed by the condensation reactions of 2-amino acids.
By referring to Table 19 of the Data Booklet, draw the structural formulas of the two dipeptides formed by the reaction of glycine with cysteine.
Describe the essential features of electrophoresis.
Arginine, cysteine and glycine undergo electrophoresis at pH 6.0. Deduce which amino acid moves towards the positive electrode (anode).
Describe what is meant by the tertiary structure of proteins.
Identify two interactions which are responsible for this type of structure.
Markscheme
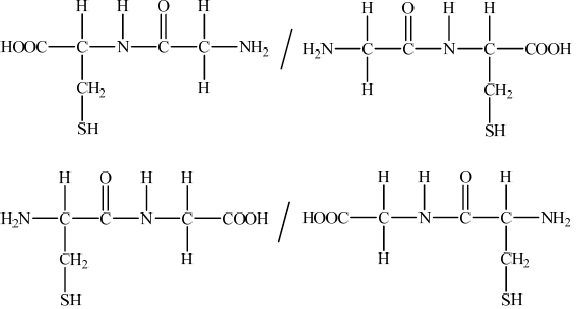
Accept condensed versions of structures such as –NH–CO–CH2– / –HN–CO–CH2–
Penalise repeated minor errors, such as incorrect representation of peptide bond (–COHN–/–NHOC–) once only.
Award [1] for a correct peptide link if the rest of the structure is incorrect.
sample of amino acids/mixture placed/spotted on gel/ polyacrylamide/PAGE/paper;
buffer solution / solution of known pH;
(high) potential (difference)/electric field/voltage applied / + and – electrodes/anode and cathode connected;
Accept current/electricity passed through.
different amino acids move different rates/distances according to their charge/isoelectric point / amino acids move towards oppositely charged electrode / OWTTE;
spray/develop with ninhydrin/organic dye/ detect by staining/fluorescence under UV light;
measure distance travelled and compare with standards/isoelectric points;
Award [1 max] for the statement “different amino-acids move to different extents”.
cysteine;
folding of secondary structure / produces 3D shape of the protein;
Do not accept “folding of the protein chain” / OWTTE.
hydrogen/H bonds
ionic bonds/attraction
van der Waals’/London/dispersion forces
disulfide bridges
hydrophobic interactions
Award [1] for any two of the above.
Examiners report
Whilst some candidates were unaware of how amino acids might join (or even what they were!) many could correctly write the structures of the two possible dipeptides. Many candidates knew the basics of electrophoresis, but the high scores they achieved were also a reflection of a generous markscheme and this lack of a sound understanding was often reflected in their inability to correctly identify the acid that would move to the anode. Whilst many candidates knew interactions responsible for the tertiary structure of proteins, their descriptions of what this was often failed to differentiate it from secondary structure.
Whilst some candidates were unaware of how amino acids might join (or even what they were!) many could correctly write the structures of the two possible dipeptides. Many candidates knew the basics of electrophoresis, but the high scores they achieved were also a reflection of a generous markscheme and this lack of a sound understanding was often reflected in their inability to correctly identify the acid that would move to the anode. Whilst many candidates knew interactions responsible for the tertiary structure of proteins, their descriptions of what this was often failed to differentiate it from secondary structure.
Whilst some candidates were unaware of how amino acids might join (or even what they were!) many could correctly write the structures of the two possible dipeptides. Many candidates knew the basics of electrophoresis, but the high scores they achieved were also a reflection of a generous markscheme and this lack of a sound understanding was often reflected in their inability to correctly identify the acid that would move to the anode. Whilst many candidates knew interactions responsible for the tertiary structure of proteins, their descriptions of what this was often failed to differentiate it from secondary structure.
Whilst some candidates were unaware of how amino acids might join (or even what they were!) many could correctly write the structures of the two possible dipeptides. Many candidates knew the basics of electrophoresis, but the high scores they achieved were also a reflection of a generous markscheme and this lack of a sound understanding was often reflected in their inability to correctly identify the acid that would move to the anode. Whilst many candidates knew interactions responsible for the tertiary structure of proteins, their descriptions of what this was often failed to differentiate it from secondary structure.
Whilst some candidates were unaware of how amino acids might join (or even what they were!) many could correctly write the structures of the two possible dipeptides. Many candidates knew the basics of electrophoresis, but the high scores they achieved were also a reflection of a generous markscheme and this lack of a sound understanding was often reflected in their inability to correctly identify the acid that would move to the anode. Whilst many candidates knew interactions responsible for the tertiary structure of proteins, their descriptions of what this was often failed to differentiate it from secondary structure.
Define the term iodine number.
Diets that are high in omega-3 fatty acids are recommended as healthy for the heart. Eicosapentaenoic acid \(({M_{\text{r}}} = 302)\) is a common omega-3 fatty acid found in fish oils. Calculate the number of carbon-carbon double bonds in one molecule of this acid if 3.02 g of this acid reacts with 12.7 g of \({{\text{I}}_{\text{2}}}{\text{ }}({M_{\text{r}}} = 254)\).
Markscheme
mass (in g) of I2/iodine reacting with 100 g of fat/oil/substance/lipid;
Allow “grams of I2” instead of “mass (in g) of I2”.
Allow amount/number of mol of I2 reacting with 1 mol of fat/oil/substance/lipid.
Do not accept mass number for mass.
\(\left( {\frac{{3.02}}{{302}} = } \right){\text{ }}0.0100{\text{ (mol)}}\) acid;
\(\left( {\frac{{12.7}}{{254}} = } \right){\text{ }}0.0500{\text{ (mol) }}{{\text{I}}_2}\);
\(\left( {\frac{{0.0500}}{{0.0100}} = } \right){\text{ }}5\) (carbon-carbon double bonds);
Award [1 max] for 5 if no working shown.
Examiners report
The better candidates gave the correct definition of iodine number, namely the mass in grams of iodine reacting with 100 g of fat. This is the precise definition of iodine number that should be emphasised to candidates in the teaching programme. Some candidates incorrectly stated amount in grams instead of mass in grams of iodine, which showed poor understanding of the inherent difference between mass, measured in grams, and amount, measured in moles. The iodine number calculation on calculating the number of C=C in eicosapentaenoic acid was well answered and full marks were typically scored.
The better candidates gave the correct definition of iodine number, namely the mass in grams of iodine reacting with 100 g of fat. This is the precise definition of iodine number that should be emphasised to candidates in the teaching programme. Some candidates incorrectly stated amount in grams instead of mass in grams of iodine, which showed poor understanding of the inherent difference between mass, measured in grams, and amount, measured in moles. The iodine number calculation on calculating the number of C=C in eicosapentaenoic acid was well answered and full marks were typically scored.
Paper chromatography is a simple method used to separate and identify the components in a mixture. To aid identification, the retention factor, \({R_{\text{f}}}\), of an unknown component can be compared with the \({R_{\text{f}}}\) values of pure samples of the possible components.
State the meaning of the term retention factor.
Explain why the value of the retention factor for the same component can be very different if different solvents (eluents) are used for the mobile phase.
If the components of the mixture are coloured then they can be seen with the naked eye. Describe two different ways in which a chromatogram can be developed if the components are colourless.
Markscheme
The ratio between the distance moved by the spot and the distance moved by the solvent front / OWTTE;
Accept this expressed as a correct equation.
\({R_{\text{f}}}\) value depends on the intermolecular forces that the component has with the mobile phase compared to the stationary phase / relative attraction of the component to mobile phase compared to the stationary phase / partition of the component between the mobile phase and the stationary phase / OWTTE;
if polarity of the solvents is different the intermolecular forces/attraction to mobile phase/partition will be different / OWTTE;
Accept “Components have different solubilities in different solvents” / OWTTE.
(viewing under) ultraviolet/UV light;
(staining with) a dye/ninhydrin/potassium manganate(VII);
(exposing to) iodine (vapour);
Accept "staining with a developing (re)agent".
Do not accept just staining.
Examiners report
Many students could correctly define “retention factor” though they frequently used the term “solute” rather than “component” and “solvent” rather than “eluent front”. Very few candidates were capable of describing, in sufficient detail to gain the second mark, how the properties of the eluent, such as its polarity or ability to engage in particular types of intermolecular bonding, affected the relative attraction of a particular component for the stationary and mobile phases, and hence the distance it would travel relative to the eluent front; indeed many said that as the distance travelled by the eluent might vary so the Rf value would vary. About half the candidates were aware of some technique to identify colourless components.
Many students could correctly define “retention factor” though they frequently used the term “solute” rather than “component” and “solvent” rather than “eluent front”. Very few candidates were capable of describing, in sufficient detail to gain the second mark, how the properties of the eluent, such as its polarity or ability to engage in particular types of intermolecular bonding, affected the relative attraction of a particular component for the stationary and mobile phases, and hence the distance it would travel relative to the eluent front; indeed many said that as the distance travelled by the eluent might vary so the Rf value would vary. About half the candidates were aware of some technique to identify colourless components.
Many students could correctly define “retention factor” though they frequently used the term “solute” rather than “component” and “solvent” rather than “eluent front”. Very few candidates were capable of describing, in sufficient detail to gain the second mark, how the properties of the eluent, such as its polarity or ability to engage in particular types of intermolecular bonding, affected the relative attraction of a particular component for the stationary and mobile phases, and hence the distance it would travel relative to the eluent front; indeed many said that as the distance travelled by the eluent might vary so the Rf value would vary. About half the candidates were aware of some technique to identify colourless components.
The food industry uses food-grade dyes and pigments to increase the appeal of food products.
The pigment associated with the olive-green colour of the outer shell of the American lobster is astaxanthin, shown below. When cooked the lobster changes to a red colour.
Identify the class of pigment to which astaxanthin belongs.
Explain why the properties of pigments in the shell of a live lobster can lead to colour variation (for example, from olive-green to orange).
Explain how the colour of astaxanthin changes to red when cooked.
Markscheme
carotenoids/carotenes;
colour masked changing light-absorption properties (resulting in colour variation) / protein holds pigment/astaxanthin tightly / protein forms a complex with the pigment/astaxanthin / OWTTE;
Do not allow protein combines with pigment.
(astaxanthin stable in heat but high temperature causes) protein to change shape/denature/uncoil/break down / OWTTE;
cartenoid pigment released from the protein (allowing red colour to appear) / other colours absorbed / OWTTE;
M2 can only be scored if M1 is correct.
Examiners report
Most candidates knew that astaxanthin was a carotenoid, but it was rare that a candidate recognized the role of the complex with the protein in altering the colour in part (b) (although it is explained in the programme guide). This was one of the most discriminating questions in the paper.
Most candidates knew that astaxanthin was a carotenoid, but it was rare that a candidate recognized the role of the complex with the protein in altering the colour in part (b) (although it is explained in the programme guide). This was one of the most discriminating questions in the paper.
Most candidates knew that astaxanthin was a carotenoid, but it was rare that a candidate recognized the role of the complex with the protein in altering the colour in part (b) (although it is explained in the programme guide). This was one of the most discriminating questions in the paper.
Vitamins are organic micronutrients essential for good health. The structures of vitamins A, C and D are given in Table 21 of the Data Booklet.
Only one of these three vitamins is soluble in water.
Vitamin D is the only vitamin that can be synthesized in the body, by the action of sunlight on the skin.
Identify by name two functional groups that are common to all three of these vitamins.
Identify this vitamin.
Explain why this vitamin is soluble in water.
State one effect of vitamin D deficiency.
Suggest why vitamin D deficiency diseases are becoming increasingly common in young people.
Markscheme
alcohol/hydroxyl group and alkene;
Accept carbon-carbon double bond.
Do not accept just double bond.
Do not accept hydroxide.
vitamin C / ascorbic acid;
several OH groups / polar molecule;
able to form hydrogen bonds with water;
softening/malfunctioning of bones / causes low/deficiency in calcium;
Accept rickets.
less time outdoors / skin not exposed due to clothing/sunscreen / OWTTE;
Accept answers that show link with outdoors/sunlight.
Examiners report
In (a) the alkenyl group was usually replaced by something else but it was pleasing to see the general absence of the answer “hydroxide”. Parts (b) and (c) were answered well and candidates seemed well aware of the dangers of modern technology keeping them indoors.
In (a) the alkenyl group was usually replaced by something else but it was pleasing to see the general absence of the answer “hydroxide”. Parts (b) and (c) were answered well and candidates seemed well aware of the dangers of modern technology keeping them indoors.
In (a) the alkenyl group was usually replaced by something else but it was pleasing to see the general absence of the answer “hydroxide”. Parts (b) and (c) were answered well and candidates seemed well aware of the dangers of modern technology keeping them indoors.
In (a) the alkenyl group was usually replaced by something else but it was pleasing to see the general absence of the answer “hydroxide”. Parts (b) and (c) were answered well and candidates seemed well aware of the dangers of modern technology keeping them indoors.
In (a) the alkenyl group was usually replaced by something else but it was pleasing to see the general absence of the answer “hydroxide”. Parts (b) and (c) were answered well and candidates seemed well aware of the dangers of modern technology keeping them indoors.
The structure of one form of vitamin E is shown below.
State and explain whether vitamin E is fat soluble or water soluble.
Markscheme
fat soluble as long non-polar/hydrocarbon chain;
with few polar groups;
cannot form hydrogen bonds with water;
Examiners report
Most candidates knew that vitamin E is fat soluble but could not explain further to achieve the marks.
State the causes of the three deficiency diseases, beriberi, goitre and pellagra.
Beriberi:
Goitre:
Pellagra:
Markscheme
Beriberi
lack/deficiency of vitamin B1/thiamine;
Goitre
lack/deficiency of iodine;
Pellagra
lack/deficiency of vitamin B3/niacin;
Examiners report
Many candidates were unable to state the causes of the deficiency diseases. Some simply guessed the same answer three times. The cause of goitre was best known of the three diseases.
Glucose is a monomer of starch.
Explain why two cyclic isomers are formed from the straight-chain glucose and name both isomers.
State the name of the two polymeric forms of starch.
Markscheme
C1 is asymmetric/chiral/has four different groups;
forming two isomers where the OH-group is on a different side (of C1/ring);
\(\alpha \)-glucose and \(\beta \)-glucose;
Accept suitable diagrams as an explanation for M2.
amylose and amylopectin;
Examiners report
Many candidates named the two isomers correctly, few could explain clearly why they are formed. Many candidates think \(\alpha \)- and \(\beta \)-glucose are optical isomers
Many candidates identified amylose and amylopectin.
Lipids are an important part of the human diet.
Fatty acids react with glycerol to form fats and oils. State the name of the chemical link formed in this reaction and the name of the other product.
The table below shows average figures for the percentage fatty acid composition of some common fats and oils.
(i) Deduce, with a reason, which fat or oil from the table above has the lowest iodine number.
(ii) Deduce, with a reason, which fat or oil from the table above is most likely to become rancid when exposed to the air.
(iii) The P/S index of a fat or oil is the ratio of polyunsaturated fat to saturated fat present. It is sometimes used to compare the relative health benefits of different lipids in the diet. Calculate the P/S index of beef fat and soybean oil.
(iv) Suggest why a P/S index of greater than 1 is considered beneficial to health.
(v) Cotton seed oil and corn oil have similar iodine numbers but the melting point of cotton seed oil is higher than that of corn oil. Suggest an explanation in terms of the structure and bonding in these two oils.
Markscheme
Name of the chemical link: ester/ethoxycarbonyl
AND
Name of the other product: water
Do not accept formulas.
Do not accept “esterification”
i
coconut oil AND lowest «percentage of» unsaturated fatty acids
OR
coconut oil AND smallest number of C=C bonds
OR
coconut oil AND highest «percentage of» saturated fatty acids
Accept “fats” for “fatty acids”.
ii
soybean oil AND highest «percentage of» polyunsaturated fatty acids
OR
soybean oil AND greatest number of C=C bonds
OR
soybean oil AND lowest «percentage of» saturated fatty acids
Accept “fats” for “fatty acids”.
iii
Beef fat: «P/S = \(\frac{3}{{59}}\) = » 0.05
AND
Soybean oil: «P/S = \(\frac{{50 + 8}}{{14}}\) =» 4.1
iv
«higher proportion of» polyunsaturated fatty acids decrease risk of atherosclerosis/heart disease/cardiovascular disease/CVD
OR
«higher proportion of» polyunsaturated fatty acids which are less likely to be deposited on the walls of arteries «than saturated fatty acids»
Accept converse arguments.
Accept correct arguments in terms of HDL and LDL but not in terms of “good” and “bad” cholesterol.
Accept “fats” for “fatty acids”.
v
Any two of:
cotton seed oil has «a higher proportion of» longer chain/greater molar mass fatty acids
molecules of cotton seed oil have greater surface area/have higher electron density
Accept “molecules of cotton seed oil are packed more closely/have more regular structure” for M2.
stronger London/dispersion/instantaneous induced dipole-induced dipole forces between chains in cotton seed oil
Accept converse arguments.
Accept “fats” for “fatty acids”.
Examiners report
A healthy diet consists of a range of food groups in the right proportions that provide the energy for the body to function, grow and repair itself.
Examples of straight-chain fatty acids include \({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{39}}}}{\text{COOH}}\), \({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}\) and \({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{29}}}}{\text{COOH}}\).
State the empirical formula and structural features of monosaccharides.
Deduce the structural formula of a triester formed from three long-chain carboxylic acid molecules, RCOOH, and one propane-1,2,3-triol molecule, \({\text{HO–C}}{{\text{H}}_{\text{2}}}{\text{CH(OH)–C}}{{\text{H}}_{\text{2}}}{\text{OH}}\). Identify one of the ester linkages in the structure by drawing a rectangle around it.
Deduce the number of C=C bonds present in one molecule of each fatty acid.
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{39}}}}{\text{COOH}}\):
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}\):
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{29}}}}{\text{COOH}}\):
Markscheme
\({\text{C}}{{\text{H}}_2}{\text{O}}\);
Accept (CH2O)n
one carbonyl/C=O and (at least two) hydroxyl/OH groups;
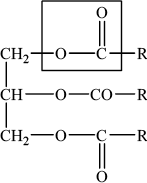
Award [1] for structure that shows unambiguously how the atoms are arranged together.
Award [1] for identifying one of the three ester linkages – must not include R and/or CH2.
\({{\text{C}}_{19}}{{\text{H}}_{39}}{\text{COOH}}\): 0
\({{\text{C}}_{19}}{{\text{H}}_{31}}{\text{COOH}}\): 4
\({{\text{C}}_{19}}{{\text{H}}_{29}}{\text{COOH}}\): 5
All three [2], any two [1], any one [0].
Examiners report
The vast majority of candidates were able to state the empirical formula of monosaccharides, but a good number were not able to state its structural features.
It was surprising to see that only about half could deduce the structure of the triester correctly and identify the ester linkage.
About half of the candidates were able to deduce the number of C=C bonds correctly from the list of fatty acids.
The building blocks of human proteins are the 2-amino acids with the general formula H2N–CHR–COOH, where R represents a side-chain specific to each amino acid. A list of these amino acids and their isoelectric points is given in table 19 of the data booklet.
State why they are called 2-amino acids.
Identify the amino acid with the empirical formula C3H7ON2
Deduce the structure of valine in a solution with a pH of 4.0.
Deduce the primary structures of the tripeptides formed by reacting together one molecule of each of the amino acids aspartic acid (Asp), glutamine (Gln) and histidine (His), using three-letter abbreviations to represent the amino acids.
Proteins carry out a number of important functions in the body. State the function of collagen.
Markscheme
NH2/amino group at C2 (while C of COOH/carboxyl/carboxylic acid is C1) / OWTTE;
Accept amine for NH2.
arginine/Arg;
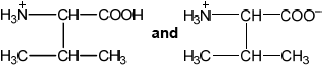 ;
;
Structural formulas of cation and zwitterion are required for the mark.
Accept structural formula of zwitterion alone (as it’s the dominant form).
Accept structural formula of cation alone (though lower in concentration than zwitterion based on equilibrium and pH calculations).
Accept full or condensed structural formula(s).
Asp–Gln–His;
Asp–His–Gln;
Gln–Asp–His;
Gln–His–Asp;
His–Asp–Gln;
His–Gln–Asp;
Award [2] for all six correct, [1] for five, four or three.
gives strength to tendons/bones/ligament/skin/cornea/cartilage/blood vessels / connective tissue;
Accept “elasticity” for “strength” but do not accept answers such as “protects bones” etc.
Accept just “structural”.
Examiners report
About a fifth of the candidates knew the reason why amino acids are called 2-amino acids. Some candidates attributed the ‘2’ to the position of the R group and others to the presence of two functional groups.
About half of the candidates were able to identify arginine as the amino acid with the empirical formula C3H7ON2.
The markscheme includes both the zwitterion and the cation, as both species exist in equilibrium at pH 4.0 (with the zwitterion having a higher concentration). Very few candidates gave the structure of the cation of valine. Some gave the structure of the zwitterion, however, the majority of candidates copied the structure of valine from the data booklet, a structure that does not exist in solution.
About a quarter of the candidates understood what was required by the question and gave the six tripeptide chains using three-letter abbreviations to represent the amino acids. Many candidates gave lots of detail for just one tripeptide.
About three quarters of the candidates stated the function of collagen correctly. Some candidates thought it provided the body with energy and others seemed to confuse it with keratin stating that it builds hair and nails.
Cholesterol is in our diet and is produced in the body. It is used to produce steroid hormones and is important in membrane structures.
Aldosterone is one of the steroid hormones produced in the body from cholesterol.
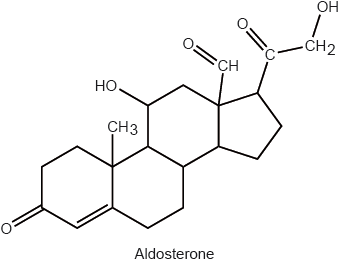
The structure of cholesterol is shown in table 21 of the data booklet. Compare the structures of cholesterol and aldosterone by naming two functional groups present in both and two functional groups present only in aldosterone.
Markscheme
Present in both:
alkenyl and hydroxyl;
Accept alkene and alcohol/hydroxy but not hydroxide.
Present only in aldosterone:
carbonyl and aldehyde;
Accept ketone (for carbonyl).
Accept primary hydroxyl/alcohol.
Award [1 max] if correct formulas, rather than names, given for both.
Examiners report
Many students were able to name the functional groups in aldosterone but often were not able to identify both of the functional groups present in both steroid hormones. There were a fair number of references to the ‘hydroxide’ group instead of hydroxyl. The endocrine gland was often not correctly identified although the function of progesterone of testosterone was often answered correctly albeit not always precisely.
Foods such as rice, bread and potatoes are rich in carbohydrates. There are three main types of carbohydrate – monosaccharides, disaccharides and polysaccharides.
Glucose, \({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}\), is a monosaccharide. When 0.85 g of glucose was completely combusted in a calorimeter, the temperature of 200.10 g of water increased from 20.20 °C to 27.55 °C. Calculate the energy value of glucose in \({\text{J}}\,{{\text{g}}^{ - 1}}\).
(i) Draw the straight chain structure of glucose.
(ii) Draw the structural formula of \(\alpha \)-glucose.
(iii) Distinguish between the structures of \(\alpha \)- and \(\beta \)-glucose.
(iv) Two \(\alpha \)-glucose molecules condense to form the disaccharide maltose. Deduce the structure of maltose.
One of the major functions of carbohydrates in the human body is as an energy source. State one other function of a carbohydrate.
Markscheme
\(\Delta T = 7.35\) (K/°C);
\(q( = mc\Delta T = {\text{200.10 g}} \times {\text{4.18 J}}\,{{\text{g}}^{ - 1}}{{\text{K}}^{ - 1}} \times {\text{7.35 K}}) = 6.15 \times {10^3}{\text{ J}}\) (per 0.85 g of glucose heated);
energy value \( = 7.2 \times {10^3}{\text{ (J}}\,{{\text{g}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
(i) 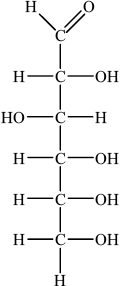
Accept CHO, CH2OH and OH groups on either side of the carbon chain provided OH on C3 is on the opposite side to OHs on C2, C4 and C5.
(ii) 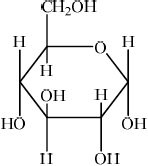 ;
;
Accept CH2OH and OH groups on either face, as long as OH on C3 is on the opposite face to the OH’s on C1, C2 and C4.
No mark awarded if HOCH2 is written, with H bonded to C or if HO is written for hydroxyl groups, with H bonded to C. Penalize this once only in (i), (ii) and (iv).
(iii) the OH on carbon-1/C-1 is inverted / difference in position of OH on carbon-1/C-1;
(iv) 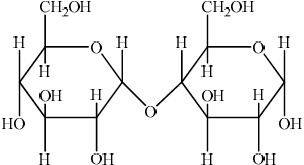 ;
;
energy reserve / can act as precursors in large number of metabolic reactions/for other biologically important molecules;
Examiners report
In (a) SD’s proved the major issue. Most candidates scored the mark for \(\Delta T\). The candidates who struggled made the following errors: incorrect mass (0.85 g) of water was used in \(q = mc\Delta T\), and failure to convert to J per g by dividing \(q\) by 0.85.
In (b)(i) many candidates struggled to draw the straight chain structure of glucose. In many instances the –OH groups were positioned incorrectly and some candidates were careless with bonding writing ‘-C–HO’ rather than ‘-C–OH’.
In (ii) a significant number of candidates mixed up the positions of the substituents on the two faces. In (iii) C1 was commonly omitted. It was surprising to see that quite a few candidates could not draw the structure of maltose in (iv). The most common mistake involved an incorrect linkage
Part (c) was answered well by the vast majority.
Rancidity limits the shelf life of foods containing oils and fats.
Rancidity can occur as a result of two separate processes. State these processes and explain the difference between them.
Markscheme
hydrolytic rancidity and oxidative rancidity;
hydrolytic rancidity involves (reaction with water) breaking ester bond / formation of a fatty acid and glycerol / OWTTE;
oxidative rancidity involves reaction of carbon-carbon double bond/C=C with oxygen / addition reaction with oxygen;
Examiners report
The majority of candidates struggled in part (a) where the difference between hydrolytic and oxidative rancidity was seldom written correctly.
The following products result from the hydrolysis of a triglyceride.
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}\) \({{\text{C}}_{{\text{13}}}}{{\text{H}}_{{\text{27}}}}{\text{COOH}}\) \({{\text{C}}_{{\text{15}}}}{{\text{H}}_{{\text{29}}}}{\text{COOH}}\)
Draw a possible structure for the triglyceride.
State the other reactant and one essential condition that would favour this hydrolysis reaction in the body.
Identify which product is polyunsaturated, and outline why foods containing this type of fatty acid are important for health.
People who live in very cold regions need a diet with a higher ratio of fat to carbohydrate than people who live in warmer climates. Suggest why this is the case.
Markscheme
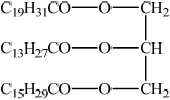 ;
;
Accept alternative orders for the hydrocarbon tails.
water/\({{\text{H}}_{\text{2}}}{\text{O}}\) and enzyme/biological catalyst/lipase;
Accept acidic/alkaline/basic condition instead of water.
Do not award mark for lipase alone without water/ H2O.
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}\);
they lower level of LDL cholesterol/low-density lipoproteins / reduce (the risk of) heart disease;
Allow comparison with saturated fats with explanation.
fats provide more energy (per kg) than carbohydrates;
Do not allow “fat is an insulator”.
Examiners report
It was clearly a challenge for candidates to reconstruct the triglyceride and in (b) conditions for the formation (rather than hydrolysis) of the triglyceride were given. Careful reading of questions is important. If water was given in (b), the enzyme was omitted or replaced by “heat”. In (c), the unsaturated product was often given in place of the polyunsaturated and only cholesterol was discussed rather than LDL cholesterol. The common error in (d) was to state that lipids “provide better insulation”.
It was clearly a challenge for candidates to reconstruct the triglyceride and in (b) conditions for the formation (rather than hydrolysis) of the triglyceride were given. Careful reading of questions is important. If water was given in (b), the enzyme was omitted or replaced by “heat”. In (c), the unsaturated product was often given in place of the polyunsaturated and only cholesterol was discussed rather than LDL cholesterol. The common error in (d) was to state that lipids “provide better insulation”.
It was clearly a challenge for candidates to reconstruct the triglyceride and in (b) conditions for the formation (rather than hydrolysis) of the triglyceride were given. Careful reading of questions is important. If water was given in (b), the enzyme was omitted or replaced by “heat”. In (c), the unsaturated product was often given in place of the polyunsaturated and only cholesterol was discussed rather than LDL cholesterol. The common error in (d) was to state that lipids “provide better insulation”.
It was clearly a challenge for candidates to reconstruct the triglyceride and in (b) conditions for the formation (rather than hydrolysis) of the triglyceride were given. Careful reading of questions is important. If water was given in (b), the enzyme was omitted or replaced by “heat”. In (c), the unsaturated product was often given in place of the polyunsaturated and only cholesterol was discussed rather than LDL cholesterol. The common error in (d) was to state that lipids “provide better insulation”.
Myoglobin is a globular protein found in the muscle tissue and is formed from 2-amino acids.
Describe the characteristic properties of 2-amino acids.
State the name of the bond or interaction that is responsible for linking the amino acids together in the primary structure.
State the name of the bond or interaction that is responsible for the secondary structure.
State two of the bonds or interactions responsible for the 3D shape of myoglobin.
Markscheme
isoelectric point;
zwitterion/forms neutral ion when \({{\text{H}}^ + }\) transfers from COOH to \({\text{N}}{{\text{H}}_{\text{2}}}\);
buffer action/can accept \({{\text{H}}^ + }\) and OH–;
general formula \({\text{HOOCCRHN}}{{\text{H}}_{\text{2}}}\);
peptide/amide;
hydrogen bonds;
hydrogen bonds;
disulfide bridges/bonds;
ionic interactions/bonds;
van der Waals’ forces / hydrophobic interactions / London/dispersion forces / temporary induced dipoles;
Examiners report
Apart from the general formula in (a), many candidates had difficulty providing the characteristic properties of 2 – amino acids. The characteristic properties are clearly identified in the syllabus details.
Most candidates could name the correct bond types in part (b).
Most candidates could name the correct bond types in part (b).
Most candidates could name the correct bond types in part (b).
Malnutrition can be caused by starvation, dieting or a person eating an excess of highly processed food.
Describe the structural composition of the following nutrients:
fats and oils
monosaccharides.
Liver is a source of arachidonic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{4}}}{{\text{(CH=CHC}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{4}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{2}}}{\text{COOH}}\), and fish oils are a source of linolenic acid. With reference to the structure of linolenic acid in Table 22 of the Data Booklet, explain why arachidonic acid has a much lower melting point compared to linolenic acid, even though it contains two more carbon atoms.
Markscheme
(tri)esters/contains COO group/(tri)glycerides;
(three) fatty acid chains joined to glycerol/propan-123-triol / OWTTE;
Accept long-chain carboxylic acid and glycerine.
empirical formula is \({\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}\) / general formula is \({{\text{C}}_n}{{\text{H}}_{{\text{2}}n}}{{\text{O}}_n}\);
contains one carbonyl/C=O group and at least two/several hydroxyl/OH groups;
four C=C bonds in arachidonic acid and three C=C bonds in linolenic acid / greater unsaturation/number of C=C bonds in arachidonic acid;
presence of double bonds prevents close-packing/kinks in structure / extra double bond decreases ability of arachidonic acid molecules to align themselves together / OWTTE;
(so) van der Waals’/London/dispersion/intermolecular forces weaker in arachidonic acid;
Examiners report
The descriptions of structure in (b) often lacked the essential points - perhaps some candidates did not know how to interpret “structural composition”.
the descriptions of structure in (b) often lacked the essential points - perhaps some candidates did not know how to interpret “structural composition”.
The effect of double bonds on the intermolecular forces in unsaturated fats was well known in (c), and thankfully few candidates referred to the breaking of covalent bonds.
Give the general structural formula for a fat or oil and describe the difference in structure between a saturated and an unsaturated fatty acid.
Explain why unsaturated fats have a lower melting point than saturated fats.
Oils can be hydrogenated. One possible problem is that partial hydrogenation may occur which produces an oil containing trans fatty acids. Explain the structural difference between a cis fatty acid and a trans fatty acid and state one disadvantage of ingesting oils containing trans fatty acids.
Difference:
Disadvantage:
Markscheme
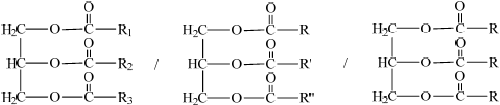 ;
;
saturated fatty acids contain only single bonds between carbon atoms/C–C whereas unsaturated fatty acids contain at least one double bond between carbon atoms/C=C / OWTTE;
the double (C=C) bond in unsaturated fats causes a “kink” so the molecules cannot pack so closely / OWTTE;
the weaker van der Waals/intermolecular forces between the molecules cause unsaturated fats to have lower melting points;
Difference
in cis the R groups on either side of the C=C point in the same direction and in trans the R groups point in opposite directions / OWTTE /
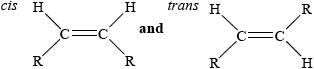 ;
;
Disadvantage
hard to metabolize;
accumulate in fatty tissue;
difficult to excrete;
increase levels of LDL;
not a good source of energy;
Award [1] for any one of the above points.
Examiners report
Few candidates knew the basic structure of a fat or oil in part (a), with the ester linkage frequently missing from the structures drawn. Describing the difference between a saturated and an unsaturated fatty acid was reasonably well answered, although often the strength of the van der Waals‟ forces was not mentioned.
In part (b) most candidates could explain why the melting point of unsaturated fats is lower than that of saturated fats.
Many candidates struggled to adequately describe the structural difference between cis and trans fatty acids in part (c). A simple diagram would have been sufficient. The disadvantage of consuming oils containing trans fatty acids was generally answered well, although some weaker candidates resorted to stating that they were bad for our health. Again, this reflects the difficulties some candidates experience in providing responses with sufficient detail.
Unsaturated fats contain C=C double bonds. The amount of unsaturation in a fat or oil can be determined by titrating with iodine solution.
(a) Define the term iodine number.
(b) Linoleic acid (\({M_{\text{r}}} = 281\)) has the following formula.
\({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{4}}}{\text{CH=CHC}}{{\text{H}}_{\text{2}}}{\text{CH=CH(C}}{{\text{H}}_{\text{2}}}{{\text{)}}_{\text{7}}}{\text{COOH}}\)
Calculate the volume of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) iodine solution required to react exactly with 1.00 g of linoleic acid.
Markscheme
(a) the number of grams/mass of iodine that add to/react with 100 g of the fat/lipid/oil;
(b) amount of linoleic acid \( = \frac{1}{{281}} = 0.00356{\text{ (mol)}}\);
amount of \({{\text{I}}_{\text{2}}}\) required \( = 2 \times 0.00356 = 0.00712{\text{ (mol)}}\);
volume of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution \( = 7.12{\text{ c}}{{\text{m}}^{\text{3}}}{\text{/0.00712 d}}{{\text{m}}^{\text{3}}}\);
OR
281 g of acid require 507.6 g of iodine;
1 g of acid requires 1.806 g/0.00712 mol of iodine;
volume of \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution \( = 7.12{\text{ c}}{{\text{m}}^{\text{3}}}{\text{/0.00712 d}}{{\text{m}}^{\text{3}}}\);
Award [3] for correct final answer.
Examiners report
In part (a), many candidates failed to score a mark for defining iodine number as they stated it is the amount of iodine, rather than the mass of iodine. The Chemistry guide clearly states in 1.1.2 that amount means the number of moles of a substance. Few candidates could calculate the volume of iodine solution required in part (b). Few recognised that 2 moles of iodine reacts with each mole of linoleic acid. Many tried to use the volume of one mole of gas to find the volume of solution. This is a standard question that is clearly in the Chemistry Guide in B.4.5.
Sugars exist in both straight chain and ring forms.
Biodegradable plastics produced from starch present one solution to the environmental problem created by the use of large quantities of plastics.
Deduce the straight chain structure of ribose from its ring structure drawn in section 34 of the data booklet.
Using the partial structure given, complete the structural formula of the molecule formed from the condensation of two cyclic \(\alpha \)-glucose molecules.
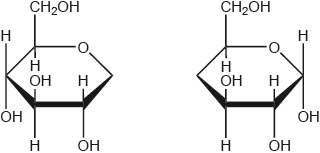
Constructing models that allow visualizations of the stereochemistry of carbohydrates is essential to understand their structural roles in cells.
Describe how Haworth projections help focus on the position of attached groups.
State one advantage of starch based polymers besides being biodegradable.
Biodegradable boxes made from polylactic acid, PLA, disintegrate when exposed to water.
State the formula of the product formed when water reacts with PLA.
Markscheme
All OH groups must be on the same side.
Accept structures with chiral carbon atoms shown as C or C* instead of crosses.
[1 mark]
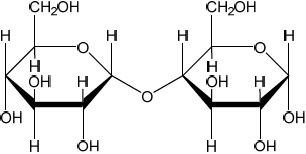
Accept –O– in a straight line provided both H’s are above the plane.
[1 mark]
«allow» 3-D perspective of structures «of cyclic monosaccharide molecules»
OR
«show» cis/same side arrangement of «attached» groups
OR
«show» trans/opposite side arrangement of «attached» groups
OR
«make» carbon and hydrogen implicit
[1 mark]
abundant/renewable/allows use of «local» vegetation
OR
less use of fossil fuel/oil based plastics
OR
air permeable/better breathing of products
OR
«can be» mixed/blended with synthetic polymers
Do not accept answers related to biodegradable examples.
Ignore any reference to cost.
Accept “carbon neutral/do not contribute to global warming”.
Accept “require less energy to produce”.
Accept “do not produce toxic products”.
[1 mark]
HO–CH(CH3)–COOH/CH3CH(OH)COOH
Do not accept C3H6O3.
Do not accept OH–CH(CH3)–COOH.
[1 mark]
Examiners report
Sunflower oil contains stearic, oleic and linoleic fatty acids. The structural formulas of these acids are given in section 34 of the data booklet.
Explain which one of these fatty acids has the highest boiling point.
10.0 g of sunflower oil reacts completely with 123 cm3 of 0.500 mol\(\,\)dm–3 iodine solution. Calculate the iodine number of sunflower oil to the nearest whole number.
Markscheme
stearic acid AND chain has no kinks/more regular structure
OR
stearic acid AND it has straight chain
OR
stearic acid AND no C=C/carbon to carbon double bonds
OR
stearic acid AND saturated
OR
stearic acid AND chains pack more closely together
stronger London/dispersion/instantaneous induced dipole-induced dipole forces «between molecules»
Accept “stearic acid AND greater surface area/electron density”.
M2 can only be scored if stearic acid is correctly identified.
Accept “stronger intermolecular/van der Waals’/vdW forces”.
[2 marks]
«n(I2) = 0.123 dm3 x 0.500 mol\(\,\)dm–3 =» 0.0615 «mol»
«m(I2) = 0.0615 mol x 253.8 g\(\,\)mol–1 =» 15.6 «g»
«iodine number \( = \frac{{15.6{\text{ g}} \times 100}}{{10.0{\text{ g}}}}\)» = 156
Award [3] for correct final answer.
Iodine number must be a whole number.
Award [2 max] for 78.
[3 marks]
Examiners report
The wavelength of visible light lies between 400 and 750 nm. The absorption spectrum of a particular anthocyanin is shown below.
Explain why pigments such as anthocyanins are coloured.
(i) Explain what effect, if any, the absorption at 375 nm will have on the colour of the anthocyanin.
(ii) Explain what effect, if any, the absorption at 530 nm will have on the colour of the anthocyanin.
List two factors which could alter the precise colour of a particular anthocyanin.
Markscheme
pigments absorb visible light;
and scatter/reflect/transmit the remaining light;
(i) no effect as it lies outside the visible region/is in the UV / OWTTE;
(ii) the colour will be the complementary colour to the colour absorbing at 530 nm / it will be red as 530 nm is blue-green / OWTTE;
oxidation;
temperature;
pH/acidity/basicity;
presence of metal ions;
Examiners report
Many candidates failed to mention that visible light is absorbed by coloured pigments and that the complimentary colour is seen.
Part (b) was answered correctly by most candidates, although some neglected to refer to their Data Booklet to identify the regions of the spectrum.
The majority of candidates could list factors which alter the precise colour of a particular anthocyanin in part (c).
Lipids play a significant role in human nutrition and have many important biological functions. The triglycerides are one type of lipid.
Table 22 of the Data Booklet shows the formulas of some fatty acids.
Olive oil contains a triglyceride (glyceryl trioleate) which, on hydrolysis, yields propane-1,2,3-triol (glycerol) and oleic acid.
Deduce the equation for this reaction. You may use the letter R to represent the hydrocarbon chains.
Calculate the iodine number for oleic acid (\({M_{\text{r}}}\) of oleic acid \( = 282.52\)).
Linoleic acid and stearic acid have similar molecular masses. Explain why linoleic acid has a much lower melting point than stearic acid.
Linoleic acid and linolenic acid are classed as essential fatty acids. State the importance of these fatty acids in the human diet.
Markscheme
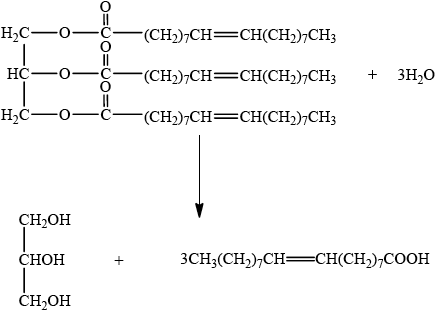
correct structure for triglyceride;
correct structures for products;
correct balancing;
Mark for balancing can only be awarded if reactants and products are correct.
Accept more condensed structural formula, but ester group must be the correct
way round (glycerol–OOC–R or glycerol–O–CO–R, not glycerol–COO–R).
Do not penalize minor errors in the hydrocarbon chain or in the use of R.
100 g of oleic acid reacts with \(\frac{{{\text{253.8}} \times {\text{100}}}}{{{\text{282.52}}}}{\text{ (g)}}\) of \({{\text{I}}_{\text{2}}}\);
Do not penalize use of integer values for \({M_r}\).
hence iodine number is 89.9;
Accept answers between 89.7 and 90.
Award [2] for for correct answer.
Award [1] for an iodine number between 44.8 and 45.0 if monatomic iodine is assumed.
Award [1] for an iodine number between 0.897 and 0.900 if 1 g of oleic acid assumed.
Award [1] for an iodine number between 111 and 112 – mass of I2 reacting with 100 g of oleic acid.
in linoleic acid, presence of C=C/double bond/unsaturation prevents close packing/leads to kinks/bends in chain;
Do not allow mark without reference to C=C/double bond/unsaturation.
hence weaker van der Waals’/London/dispersion forces between molecules;
Accept opposite statements for stearic acid but must point out that this is because it does not have a C=C/double bond/unsaturation.
cannot be synthesized by body;
Accept specific uses such as lowers LDL cholesterol level, increases HDL
cholesterol level or lowers risk of heart disease.
Do not accept just “lowers cholesterol level”.
Examiners report
This proved surprisingly challenging. Even using R for the hydrocarbon chain, many candidates found drawing the structure of a triglyceride a challenge and only a handful correctly balanced the equation by adding an appropriate number of water molecules. From the way in which the calculation was tackled, very few knew the definition of iodine number and there were even less correct answers. Few students gained marks for the early steps of the calculation because their working was rarely clear. The effect of the double bond on packing was better known, as was the importance of essential fatty acids.
This proved surprisingly challenging. Even using R for the hydrocarbon chain, many candidates found drawing the structure of a triglyceride a challenge and only a handful correctly balanced the equation by adding an appropriate number of water molecules. From the way in which the calculation was tackled, very few knew the definition of iodine number and there were even less correct answers. Few students gained marks for the early steps of the calculation because their working was rarely clear. The effect of the double bond on packing was better known, as was the importance of essential fatty acids.
This proved surprisingly challenging. Even using R for the hydrocarbon chain, many candidates found drawing the structure of a triglyceride a challenge and only a handful correctly balanced the equation by adding an appropriate number of water molecules. From the way in which the calculation was tackled, very few knew the definition of iodine number and there were even less correct answers. Few students gained marks for the early steps of the calculation because their working was rarely clear. The effect of the double bond on packing was better known, as was the importance of essential fatty acids.
This proved surprisingly challenging. Even using R for the hydrocarbon chain, many candidates found drawing the structure of a triglyceride a challenge and only a handful correctly balanced the equation by adding an appropriate number of water molecules. From the way in which the calculation was tackled, very few knew the definition of iodine number and there were even less correct answers. Few students gained marks for the early steps of the calculation because their working was rarely clear. The effect of the double bond on packing was better known, as was the importance of essential fatty acids.
Many factors affect the shelf life of food products.
Describe the rancidity of fats.
Markscheme
(perception of flavours in lipids due to) disagreeable smell/taste/texture/appearance / OWTTE;
Examiners report
Many candidates missed the command term used in part (c) and failed to describe the rancid food.
Genetically modified (GM) foods are now widely available, although in some countries environmental groups are campaigning against them. Define the term genetically modified food and discuss the benefits and concerns of using GM foods.
Markscheme
a genetically modified food is derived/produced from a genetically modified organism;
Award [1] for definition.
benefits:
crops: enhanced taste/quality/appearance;
reduced maturation time;
increase in nutrients and yield;
improved resistance to disease, pests and herbicides;
enrichment of rice with vitamin A;
animals: increased resistance;
productivity and feed efficiency;
better yields of milk/egg;
improved animal health;
environment: “friendly” bio-herbicides and bio-insecticides;
conservation of soil/water/energy;
improved natural waste management;
concerns:
(links to) increased allergies (for people involved in their processing);
altered composition of (balanced) diet / altered nutritional quality of food;
change in ecosystems / development of “superweeds”/“superbugs”;
Award [4 max] for benefits and concerns.
To score [4] both benefits and concerns must appear in answer.
Examiners report
Most candidates recognized that genetically modified foods come from genetically modified organisms. However, candidates generally thought of genetically modified foods as coming only from plants. Candidates did not discuss the advantages of genetic modifications in animals or for example in terms of environmental friendly pesticides.
Candidates struggled to discuss the concerns of using GM foods. Many candidates provided general and simplistic responses. For instance candidates just stated that GM foods were unnatural and therefore bad, showing no understanding of the real concerns.
Macronutrients and micronutrients are essential components of a balanced diet.
State the difference between macronutrients and micronutrients.
Markscheme
micronutrients required in much smaller quantities/very small amounts/less than 0.005% of body mass;
Accept opposite statement for macronutrients.
Examiners report
Most candidates gained full marks for this question and was answered quite well.
A balanced diet is needed for good health.
By comparing the structures of vitamins A, C and D given in Table 21 of the Data Booklet, state and explain which of the three vitamins is most soluble in water.
Markscheme
(vitamin) C/ascorbic acid;
more/many/several hydroxyl/OH groups that can form hydrogen bonds (with water);
M2 cannot be awarded for incorrect vitamin.
Examiners report
This was generally well done, although some candidates did not refer to hydrogen bonds when explaining the solubility of vitamin C in water.
In recent years, the use of soybean oil by the food industry has increased. A significant proportion of this oil is produced from genetically modified soybeans.
Discuss two benefits and two concerns of using genetically modified foods.
Benefits:
Concerns:
Markscheme
Benefits: [2 max]
enhanced taste/quality/appearance;
longer shelf life;
reduced maturation time;
improved tolerance of drought / marginal conditions / rainfall/temperature/nutrient levels;
increase in yield/productivity/feed efficiency;
development of crops with greater amounts of nutrients/micronutrients;
more resistant to herbicides and insecticides / permit the use of more environmentally friendly herbicides and insecticides;
increased resistance to pests/disease / improved animal health;
Concerns: [2 max]
links to increased allergies (for people involved in GM food processing);
risk of changing the composition of a balanced diet/altered nutritional quality of food;
pollen from GM crops may contaminate normal crops with unknown effects;
uncertainties about long-term health effects of genetic modification of food;
Examiners report
The benefits and concerns regarding genetically modified foods seemed to be well known with almost all candidates scoring well, though a common failing was again the use of journalistic descriptions that lacked the precision one hopes would result from an in depth study of the subject.
Proteins are polymers of 2-amino acids. The structures of the common amino acids are given in Table 19 of the Data Booklet. This question refers to the two amino acids alanine and cysteine.
With reference to the isoelectric points of alanine and cysteine:
State the structural formula of cysteine as a zwitterion.
identify a pH value where both amino acids would be positively charged.
describe with a reason what pH value would be suitable to use in an electrophoresis experiment designed to separate these two amino acids in solution.
Cysteine is responsible for a specific type of intra-molecular bonding within a protein molecule. State the name of this type of interaction and outline how it is different from other interactions responsible for the tertiary structure.
State three functions of proteins in the body and include a named example for each.
Markscheme
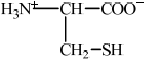 ;
;
Accept full or condensed structural formulas as long as correct charges on N/NH3 and O are represented.
Accept NH3+ for H3N+ in the diagram.
any value or range below 5.1;
any value or range from 5.1-6.0;
alanine positive and cysteine negative;
Accept biggest charge difference/opposite charges between isoelectric points so move in opposite directions.
Need reference to charges to score M2.
disulfide bridge;
Accept S–S.
covalent / strongest bond;
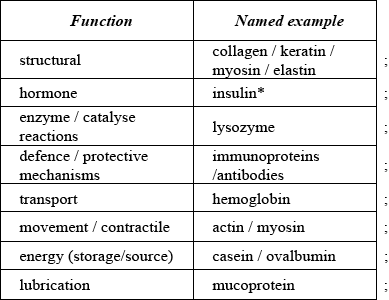
Need function with valid example for each mark.
Award [1 max] for three correct functions or three named examples.
Accept other correct examples.
Do not apply list principle.
* Other protein hormones include human growth hormone, follicle stimulating hormone (FSH), adrenocorticotropic hormone (corticotropin or ACTH), thyroid stimulating hormone (TSH).
* Do not accept steroids, sex hormones, testosterone, progesterone, estrogen, adrenalin.
Examiners report
The zwitterion mark was often lost (missing H or charge on the amine end) but pH values in (b) were correctly identified. The second mark in (b)(ii) was often lost because there was no reference to charges and the direction of migration. About half of the candidates correctly identified the disulfide bridge but fewer were able to outline the difference from other tertiary structure interactions. Part (d) caused problems for all but well-prepared candidates; they need a good grasp of subject-specific vocabulary.
The zwitterion mark was often lost (missing H or charge on the amine end) but pH values in (b) were correctly identified. The second mark in (b)(ii) was often lost because there was no reference to charges and the direction of migration. About half of the candidates correctly identified the disulfide bridge but fewer were able to outline the difference from other tertiary structure interactions. Part (d) caused problems for all but well-prepared candidates; they need a good grasp of subject-specific vocabulary.
The zwitterion mark was often lost (missing H or charge on the amine end) but pH values in (b) were correctly identified. The second mark in (b)(ii) was often lost because there was no reference to charges and the direction of migration. About half of the candidates correctly identified the disulfide bridge but fewer were able to outline the difference from other tertiary structure interactions. Part (d) caused problems for all but well-prepared candidates; they need a good grasp of subject-specific vocabulary.
The zwitterion mark was often lost (missing H or charge on the amine end) but pH values in (b) were correctly identified. The second mark in (b)(ii) was often lost because there was no reference to charges and the direction of migration. About half of the candidates correctly identified the disulfide bridge but fewer were able to outline the difference from other tertiary structure interactions. Part (d) caused problems for all but well-prepared candidates; they need a good grasp of subject-specific vocabulary.
The zwitterion mark was often lost (missing H or charge on the amine end) but pH values in (b) were correctly identified. The second mark in (b)(ii) was often lost because there was no reference to charges and the direction of migration. About half of the candidates correctly identified the disulfide bridge but fewer were able to outline the difference from other tertiary structure interactions. Part (d) caused problems for all but well-prepared candidates; they need a good grasp of subject-specific vocabulary.
Hormones play an important role in the body.
(a) Outline the function and production of hormones in the body.
(b) In many communities there are people who use steroids appropriately, and others who abuse them. Outline one appropriate use and one abuse of steroids.
Markscheme
(a) chemical messenger;
secreted directly into the blood by endocrine glands;
Accept pituitary gland, pancreas, ovaries, testes, thyroid gland and adrenal gland.
(b) build up depleted muscle due to lack of activity;
assist in recuperation from an illness;
stimulation of bone marrow;
treatment of delayed male puberty;
male contraceptive;
treatment of female-to-male gender changes;
treatment of inflammation;
Award [1] for any one use.
muscle/strength build up for an unfair advantage in sport by athletes;
Award [1] for abuse.
Examiners report
Generally well done, though a number of candidates appeared a little confused about where hormones were produced and the fact that they are released directly into the bloodstream. In Part (b) a number of candidates failed to point out that many steroid uses and its abuse are associated with muscle development.
The following diagram represents a thin-layer chromatogram of an amino acid.
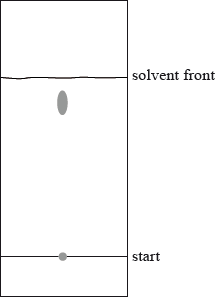
Calculate the \({R_{\text{f}}}\) of the amino acid.
Markscheme
\({R_{\text{f}}} = \frac{{40{\text{ (mm)}}}}{{46{\text{ (mm)}}}} = 0.87\);
Allow 0.86 to 0.88.
No mark if \({R_{\text{f}}}\) has units.
Examiners report
There were difficulties in the calculation of \({R_{\text{f}}}\), some candidates did not seem to know what it means, others what to measure.
Describe the chemical composition of a triglyceride.
The following two structures represent isomers of a fatty acid.
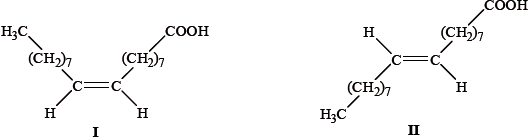
State and explain which isomer has the higher melting point.
Markscheme
ester of glycerol/propan-1,2,3-triol and three fatty acids/long chain carboxylic acids;
II;
more straight molecule/greater surface area hence greater distortion of electron cloud / allows closer packing of fatty acids for trans / does not allow closer packing for cis isomers / OWTTE;
trans greater van der Waals’ forces / cis less van der Waals’ forces;
Accept London/dispersion forces.
Examiners report
Many candidates knew the structure of triglycerides.
Most candidates identified structure II as the one with the highest melting point and many gave the correct reason.
Monosaccharides and disaccharides are classes of carbohydrates.
Describe the structural features of monosaccharides.
Draw the structures of \(\alpha \)-glucose and \(\beta \)-glucose.
\[\alpha {\text{ - glucose}}\quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \quad \beta {\text{ - glucose}}\]
Two \(\alpha \)-glucose molecules condense to form the disaccharide maltose. Draw the structure of maltose.
Markscheme
contain carbonyl (group)/C=O;
have at least two hydroxyl/OH (groups);
Do not accept hydroxide instead of hydroxyl.
\({\text{C}}{{\text{H}}_{\text{2}}}{\text{O}}\) empirical formula;
Do not accept CxH2yOy or C6H12O6.
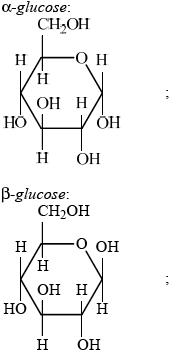
Award [1 max] if position of OH is correct in both structures at C1 but other groups/moieties (eg, CH2OH) or H are missing.
Award [1 max] if the alpha and beta structures are labelled in reverse.
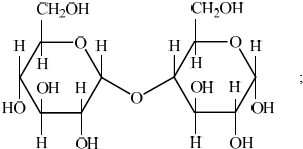
Examiners report
Only some of the candidates described a monosaccharide correctly in part (a) although its features are given in the programme guide. Drawing alpha and beta glucose was also a discriminating question.
About a third of candidates were able to score full marks on part (b)(i).
Similarly, drawing maltose in part (d)(ii) was a challenge for the majority of candidates. Many had missing hydrogen atoms or an incorrect orientation of bonds around the linkage.
The formula of linoleic acid is given in Table 22 of the Data Booklet.
Identify the structural formula of the triglyceride formed when three molecules of linoleic acid react with one molecule of glycerol (propane-1,2,3-triol), \({\text{C}}{{\text{H}}_{\text{2}}}{\text{OHCHOHC}}{{\text{H}}_{\text{2}}}{\text{OH}}\).
State the other product formed during this reaction.
Explain why the triglyceride formed from linoleic acid and glycerol is a liquid and not a solid at room temperature.
Describe how the triglyceride formed from linoleic acid and glycerol could be converted into a saturated fat and give any necessary conditions.
Other than the fact that it is a solid at room temperature, discuss two advantages and two disadvantages of a saturated fat compared to an unsaturated fat or oil.
Advantages:
Disadvantages:
Markscheme
 ;
;
Do not accept R for C17H31.
Penalize for incorrect bond connectivity.
water/\({{\text{H}}_{\text{2}}}{\text{O}}\);
double bonds cause a “kink” in the hydrocarbon chain / unsaturated hydrocarbon chains cannot pack so closely together (as saturated);
attractive forces/London/dispersion/van der Waals/vdW/LDF/ /instantaneous/temporary induced dipole-induced dipole forces between the molecules are weaker / less energy required to overcome the attraction between the molecules;
(d) addition of hydrogen/\({{\text{H}}_{\text{2}}}\) / hydrogenation;
heat and catalyst/Zn/Cu/Ni/Pd/Pt;
Accept any temperature in range 140–225 °C.
Advantages:
decreases rate of oxidation / makes it more stable / slows rancidification / has longer shelf life;
greater energy released per gram / OWTTE;
controls hardness/plasticity/stiffness;
Disadvantages:
increase risk of heart disease / increase low-density/LDL cholesterol;
does not contain essential/omega-3/omega-6 fatty acids;
hydrogenated fats might contain trans-fatty acids;
Examiners report
Generally candidates struggled with the details of the triglyceride structure; very few were able to gain the mark for part (a). Almost all the candidates were able to state water as the other product and gain the mark in part (b). Candidates often missed the specific details for the answer to part (c); they were able to identify the presence of double bonds or unsaturation but had difficulty expanding and linking the idea to how this changed packing and intermolecular forces. For part (d) many candidates stated hydrogenation as the answer and scored one point but many were not able to state the two reaction conditions correctly. Most responses on advantages and disadvantages of saturated fats, part (e), were too general for example, affects your health. A common statement was ‘better storage/ transport’ as an advantage for saturated fats. Most candidates only scored one or two points for this question.
Generally candidates struggled with the details of the triglyceride structure; very few were able to gain the mark for part (a). Almost all the candidates were able to state water as the other product and gain the mark in part (b). Candidates often missed the specific details for the answer to part (c); they were able to identify the presence of double bonds or unsaturation but had difficulty expanding and linking the idea to how this changed packing and intermolecular forces. For part (d) many candidates stated hydrogenation as the answer and scored one point but many were not able to state the two reaction conditions correctly. Most responses on advantages and disadvantages of saturated fats, part (e), were too general for example, affects your health. A common statement was ‘better storage/ transport’ as an advantage for saturated fats. Most candidates only scored one or two points for this question.
Generally candidates struggled with the details of the triglyceride structure; very few were able to gain the mark for part (a). Almost all the candidates were able to state water as the other product and gain the mark in part (b). Candidates often missed the specific details for the answer to part (c); they were able to identify the presence of double bonds or unsaturation but had difficulty expanding and linking the idea to how this changed packing and intermolecular forces. For part (d) many candidates stated hydrogenation as the answer and scored one point but many were not able to state the two reaction conditions correctly. Most responses on advantages and disadvantages of saturated fats, part (e), were too general for example, affects your health. A common statement was ‘better storage/ transport’ as an advantage for saturated fats. Most candidates only scored one or two points for this question.
Generally candidates struggled with the details of the triglyceride structure; very few were able to gain the mark for part (a). Almost all the candidates were able to state water as the other product and gain the mark in part (b). Candidates often missed the specific details for the answer to part (c); they were able to identify the presence of double bonds or unsaturation but had difficulty expanding and linking the idea to how this changed packing and intermolecular forces. For part (d) many candidates stated hydrogenation as the answer and scored one point but many were not able to state the two reaction conditions correctly. Most responses on advantages and disadvantages of saturated fats, part (e), were too general for example, affects your health. A common statement was ‘better storage/ transport’ as an advantage for saturated fats. Most candidates only scored one or two points for this question.
Generally candidates struggled with the details of the triglyceride structure; very few were able to gain the mark for part (a). Almost all the candidates were able to state water as the other product and gain the mark in part (b). Candidates often missed the specific details for the answer to part (c); they were able to identify the presence of double bonds or unsaturation but had difficulty expanding and linking the idea to how this changed packing and intermolecular forces. For part (d) many candidates stated hydrogenation as the answer and scored one point but many were not able to state the two reaction conditions correctly. Most responses on advantages and disadvantages of saturated fats, part (e), were too general for example, affects your health. A common statement was ‘better storage/ transport’ as an advantage for saturated fats. Most candidates only scored one or two points for this question.
Explain, giving their names, the two types of reaction by which foods may become rancid.
Reaction 1:
Reaction 2:
Markscheme
Reaction 1: hydrolytic (rancidity)/hydrolysis and
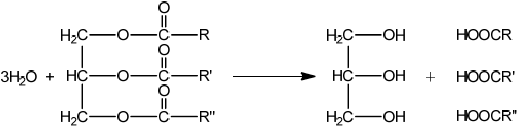
OR
hydrolytic (rancidity) and hydrolysis of ester links/breaking down of lipid/fat to glycerol/propane-1,2,3-triol and fatty/carboxylic acids;
Reaction 2: oxidative (rancidity)/oxidation and addition of O2 across C=C/carbon−carbon double bonds / oxidation of C=C/carbon−carbon double bonds;
Do not penalize omission of “carbon−carbon” if already penalized in F.17(b)(i).
Award [1 max] for “hydrolytic (rancidity)/hydrolysis” and “oxidative (rancidity)/oxidation” only.
Examiners report
Some candidates gained a mark for naming both processes by which food becomes rancid. However, candidates rarely explained the reactions correctly.
Lipids are a group of naturally occurring largely non-polar biomolecules. The term iodine number is often used to characterize particular lipids.
(i) Define the term iodine number.
(ii) A sample containing \(1.12 \times {10^{ - 2}}\) mol of fatty acid was found to react with 8.50 g of iodine, \({{\text{I}}_{\text{2}}}\). Calculate the number of carbon-carbon double bonds present in the fatty acid, showing your working.
(i) Draw the structure of glycerol (propane-1,2,3-triol).
(ii) Glycerol can react with three molecules of lauric acid to form a triglyceride.
The structure of lauric acid is given in Table 22 of the Data Booklet. State the name of the functional group of the triglyceride and identify the other product formed.
Name of functional group of triglyceride:
Other product formed:
The hydrolysis of tristearin, whose structure is shown below, can be catalysed by the enzyme lipase.
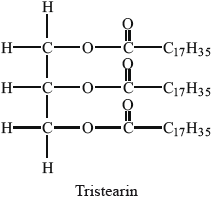
Successive hydrolysis of tristearin results in the formation of distearin and monostearin.
Deduce the structure of the diglyceride, distearin, and state the name of the other product formed from this reaction.
Structure of diglyceride, distearin:
Name of other product:
Explain why the metabolism of fats produces much more energy per gram than that of carbohydrates.
Markscheme
(i) mass (in g) of \({{\text{I}}_{\text{2}}}\) reacting with 100 g of fat/oil/substance/lipid;
Allow amount/number of mol of I2 reacting with 1 mol of fat/oil/substance/lipid.
(ii) \(\frac{{8.50}}{{253.4}}/\frac{{8.50}}{{254}}/3.35 \times {10^{ - 2}}{\text{ (mol)}}\);
\(\left( {\frac{{3.35 \times {{10}^{ - 2}}}}{{1.12 \times {{10}^{ - 2}}}}} \right) = 3\) (C=C);
OR
\(\frac{{8.50}}{{1.12}} \times {10^{ - 2}}/759\) (g \({{\text{I}}_{\text{2}}}\) react with one mol of fatty acid);
\(\left( {\frac{{759}}{{254}}} \right) = 3\) (C=C);
M2 can only be awarded if M1 is correct.
(i)  ;
;
Accept any correct representation.
(ii) Name of functional group of triglyceride:
ester
Allow triester.
Do not allow –COO–.
and
Other product formed:
water/\({{\text{H}}_{\text{2}}}{\text{O}}\)
Structure of diglyceride, distearin:
 ;
;
Accept a structure with OH in middle also.
Name of other product:
stearic acid/octadecanoic acid / stearate/octadecanoate;
Name required.
Do not allow stearin.
fats have fewer oxygens than carbohydrates (of same molar mass) / fats less oxidized;
Allow converse statements for carbohydrates.
a larger change in carbon’s oxidation number occurs when fats are oxidized / more energy is used in breaking the bonds in carbohydrates than the bonds in fats;
Examiners report
(i) About half of the candidates could define iodine number correctly. Others had the correct idea but gave imprecise answers.
(ii) About half of the candidates obtained the number of moles of iodine that reacted and gained one mark. A smaller number of candidates were able to calculate the number of double bonds in the fatty acid.
(i) Almost all candidates were able to draw the structure of glycerol.
(ii) About half of the candidates recognized the functional group in a triglyceride as an ester, and more than half of the candidates recognized water as the other product formed.
This was a discriminating question. Only a few candidates were able to deduce the structure of the diglyceride and even fewer recognized the other product as stearic acid.
Many candidates obtained a mark for stating that fats contain less oxygen or are less oxidized than carbohydrates, but were unable to score the second mark.
F and G are two synthetic hormones. The structures of some natural hormones are given in table 21 of the data booklet.
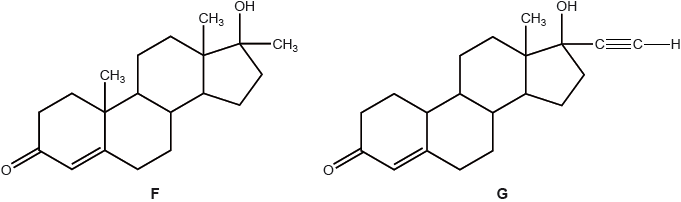
A number of famous athletes have been banned from competition for using hormone F.
Explain, with reference to its structure, why hormone F improves performance.
Markscheme
structure/function similar to testosterone;
causes increased rate of protein synthesis/tissue/muscle building/increase in muscle mass / OWTTE;
Accept “anabolic” for M2.
Examiners report
Generally well-answered. Some candidates thought hormone F was testosterone.
Olive oil is a complex mixture of triglycerides, some of which are derived from oleic acid.
State the name of the compound which combines with fatty acids to form triglycerides.
Discuss two effects on health of consuming trans fatty acids such as elaidic acid.
Markscheme
glycerol / propan-1,2,3-triol;
Accept minor errors in naming, such as propane-1,2,3-triol.
hard to metabolize / accumulates in fatty tissue / difficult to excrete;
increases levels of LDL cholesterol / increases risk of heart disease;
Do not accept “increases level of bad cholesterol”.
Examiners report
Most students correctly identified glycerol as the compound which combines with fatty acids to form triglycerides. Students often described the difference between cis and trans isomer but did not always refer to the packing of the molecules. Many candidates knew one effect on health of consuming trans fatty acids with answers often referring to the greater risk of heart disease. Common incorrect effects on health of consuming trans fatty acids were that they were "difficult to break down" or "difficult to digest". These statements are not synonymous with trans fatty acids being "hard to metabolize".
Most students correctly identified glycerol as the compound which combines with fatty acids to form triglycerides. Students often described the difference between cis and trans isomer but did not always refer to the packing of the molecules. Many candidates knew one effect on health of consuming trans fatty acids with answers often referring to the greater risk of heart disease. Common incorrect effects on health of consuming trans fatty acids were that they were "difficult to break down" or "difficult to digest". These statements are not synonymous with trans fatty acids being "hard to metabolize".
There are several types of lipids in the human body. One of these types, triglycerides, might be made of fatty acids with different degrees of saturation.
State one example of each of the following types of fatty acids (refer to Table 22 of the Data Booklet if necessary).
Saturated:
Mono-unsaturated:
Poly-unsaturated:
Describe, by completing the equation below, the condensation of glycerol and the three fatty acids named in (a) to make a triglyceride.
(i) State the names of two other types of lipids present in the human body.
(ii) Compare their composition with that of triglycerides.
Markscheme
Saturated:
octanoic / \({{\text{C}}_7}{{\text{H}}_{15}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_6}{\text{COOH}}\) /
lauric / \({{\text{C}}_{11}}{{\text{H}}_{23}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_{10}}{\text{COOH}}\) /
palmitic / \({{\text{C}}_{15}}{{\text{H}}_{31}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_{14}}{\text{COOH}}\) /
stearic / \({{\text{C}}_{17}}{{\text{H}}_{35}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_{16}}{\text{COOH}}\);
Mono-unsaturated:
oleic / \({{\text{C}}_{17}}{{\text{H}}_{33}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_7}{\text{CH=CH(C}}{{\text{H}}_2}{{\text{)}}_7}{\text{COOH}}\);
Poly-unsaturated:
linoleic / \({{\text{C}}_{17}}{{\text{H}}_{31}}{\text{COOH/C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_4}{{\text{(CH=CHC}}{{\text{H}}_2}{\text{)}}_2}{{\text{(C}}{{\text{H}}_2}{\text{)}}_6}{\text{COOH}}\) /
linolenic / \({{\text{C}}_{17}}{{\text{H}}_{29}}{\text{COOH/C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{(CH}}\)=\({\text{CHC}}{{\text{H}}_2}{{\text{)}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_6}{\text{COOH}}\);
Accept name or formula.
Accept other correct examples of fatty acids.
Accept systematic names instead of trivial names.
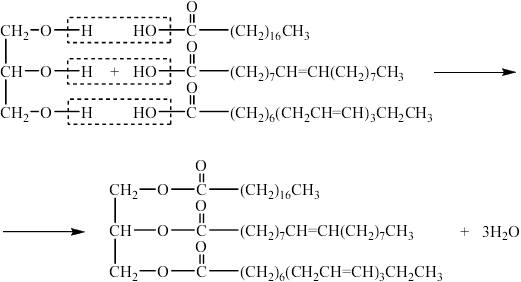
This scheme is only one of many possible examples.
the release of three molecules of water;
correct structure of all three ester groups;
Accept more condensed structural formulas.
Ester group must be written correctly, glycerol–OOC–R (not glycerol–COO–R).
Do not penalize for minor mistakes in the hydrocarbon chains or the use of R.
(i) phospholipids and steroids;
Do not accept cholesterol/other specific examples.
(ii) all three types of lipids are (predominantly) hydrophobic/non-polar/consist mostly of hydrocarbon fragments;
triglycerides and (most) phospholipids contain (a fragment of) glycerol;
steroids are (poly)cyclic compounds/contain (several) rings;
phospholipids contain phosphate (group);
triglycerides and phospholipids are esters;
Allow phosphoric acid/phosphorus instead of phosphate in phospholipids.
Allow cholesterol is (poly)cyclic compound/contains (several) rings as ECF from (i).
Examiners report
Most candidates were able to transfer the required information for part (a) correctly from the Data Booklet.
In (b), the mark for three molecules of water was more often gained than the mark for the correct drawing of the ester link which was often the wrong way round (glycerol–COO–R rather than glycerol–OOC–R) – or just wrong.
In (c), cholesterol was most often given in place of steroids. On reflection, composition might not have been the best word to use in (c)(ii), but it seemed to cause candidates little difficulty and the mark-scheme allowed candidates to score well. Candidates need to consider the best way to set out answers asking for a comparison.
The principles of chromatography can be demonstrated using paper chromatography to analyse the ink of a pen, using propanone as the mobile phase.
(a) State how you could tell whether the ink was a single substance or a mixture of components.
(b) Explain how paper chromatography separates the components.
(c) The \({R_{\text{f}}}\) value of the components of the ink could be measured. Define the term \({R_{\text{f}}}\).
(d) State one factor that would alter the \({R_{\text{f}}}\) value of a particular component.
Markscheme
(a) whether chromatogram had just one spot or number of spots / OWTTE;
Allow “component/dot / OWTTE” for spot.
(b) different components have different attractions/affinities/bond strengths/solubilities for two phases / OWTTE;
components strongly absorbed/adsorbed by stationary phase move less / components weakly absorbed/adsorbed by stationary phase move more / components not very soluble in mobile phase move less / components very soluble in mobile phase move more / OWTTE;
(c) distance travelled by component divided by distance travelled by solvent (front);
Allow spot/solute for component.
Accept Rf represented as an equation.
Do not allow just retention factor.
(d) (nature of) solvent (used) / (type of) paper / temperature / pH;
Examiners report
Parts (a), (c) and (d) were well done. In (d) some candidates stated that solubility is a factor. However solubility depends on conditions and is not a factor per se. In (b) although often candidates stated that different components have different affinities for the two phases, some did not mention the two phases and others did not refer to comparative movement to score the second mark (e.g. components very soluble in the mobile phase will travel further / OWTTE). One G2 comment stated that the command term "explain" was not appropriate for this question. This was discussed at grade award and the senior examining team felt that this indeed was the most appropriate command term for the nature of the question asked.
(a) Describe the differences in the structure between the saturated fatty acid \({{\text{C}}_{{\text{16}}}}{{\text{H}}_{{\text{32}}}}{{\text{O}}_{\text{2}}}\) and the unsaturated fatty acid \({{\text{C}}_{{\text{16}}}}{{\text{H}}_{{\text{26}}}}{{\text{O}}_{\text{2}}}\).
(b) Describe how \({{\text{C}}_{{\text{16}}}}{{\text{H}}_{{\text{26}}}}{{\text{O}}_{\text{2}}}\) can be converted to \({{\text{C}}_{{\text{16}}}}{{\text{H}}_{{\text{32}}}}{{\text{O}}_{\text{2}}}\).
(c) Fatty acids are components of fats and oils.
(i) Describe one advantage of the products formed by hydrogenating fats and oils.
(ii) Describe one disadvantage of the products formed by hydrogenating fats and oils.
Markscheme
(a) unsaturated fatty acid has (3) C=C double bonds;
saturated fatty acid has only single C–C bonds;
unsaturated fatty acid can display cis and trans isomerism / saturated fatty acid cannot display cis and trans isomerism;
saturated fatty acid chains are straighter than unsaturated chains / OWTTE;
(b) hydrogen/ 2 H /hydrogenation;
(high pressure and) high temperature/any temperature in the range of 150 °C to 200 °C /heat;
catalyst/nickel/Ni/platinum/Pt/copper/Cu/zinc/Zn;
(c) (i) semi-solid/solid fat/lipid (with higher melting point);
decreased rate of oxidation/stability increases with increasing saturation;
increased hardness;
control feel and plasticity/stiffness;
hydrogenated vegetable fats are cheaper than animal fats;
(ii) (trans fatty acids can be formed from partial hydrogenation of fats and oils)
(trans fatty acids) are difficult to metabolize/accumulate in fatty tissue/are difficult to excrete;
(trans fatty acids) increase the levels of LDL cholesterol;
(trans fatty acids) are a low quality energy source;
mono- and poly-unsaturated fats are healthier (for the heart) than saturated fats;
Examiners report
This was generally not well done, with many candidates in the first part failing to note the degree of unsaturation as well as not specifying it was carbon-carbon bonds they were referring to. In Part (b) the conditions for hydrogenation reactions appeared not to be well known, though many candidates gained some marks on the advantages and disadvantages of hydrogenated fats, though the latter often lacked the required precision.
Paper chromatography may be used to separate a mixture of sugars.
(a) State the stationary phase and an example of a mobile phase used in paper chromatography.
Stationary phase:
Mobile phase:
(b) The identity of two sugars in a mixture can be determined by measuring their \({R_{\text{f}}}\) values, after staining.
(i) Describe how an \({R_{\text{f}}}\) value can be calculated.
(ii) Calculate the \({R_{\text{f}}}\) value for sugar 2 in the chromatogram below.
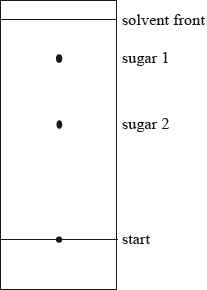
(c) Explain how the \({R_{\text{f}}}\) value of sugar 2 could be used to identify it.
Markscheme
(a) Stationary phase:
water in the paper;
Mobile phase:
water / any other non corrosive solvent or solvent mixture;
(b) (i) \({R_{\text{f}}} = \frac{{{\text{distance moved by substance}}}}{{{\text{distance moved by solvent / solvent front}}}}\);
(ii) \({R_{\text{f}}} = \frac{{3.0}}{{5.7}} = 0.53\);
Accept answers between 0.5 and 0.55 (± 1mm on measurements).
(c) compare \({R_{\text{f}}}\) of unknown to known values;
(conducted) under the same conditions (of stationary/mobile phase);
Allow second mark for specifying a particular condition to keep the same (e.g. solvent).
Examiners report
This was generally well done with most candidates displaying a good comprehension of the \({R_{\text{f}}}\) concept. In Part (c) many candidates correctly suggested comparing the \({R_{\text{f}}}\) value to that of standard samples, but very few pointed out that these must be obtained under identical conditions.
Thin-layer chromatography (TLC) is an example of adsorption chromatography.
A mixture of two organic compounds was separated by TLC using a non-polar solvent.
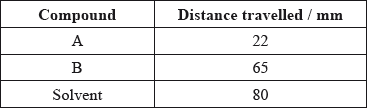
(i) Calculate the \({R_{\text{f}}}\) values of A and B.
(ii) Outline why compound B has travelled the greater distance.
Markscheme
(i)  ;
;
Award [1] for both correct.
(ii) B is more soluble in solvent/mobile phase / B is less polar than A / B is less strongly adsorbed onto stationary phase;
Accept B is non-polar.
Do not allow “greater attraction/affinity to solvent” without reference to solubility.
Examiners report
(i) Very well answered. Many candidates used an inappropriate number of significant figures for the calculated \({R_{\text{f}}}\) values, but this was not penalized in this instance.
(ii) Generally well answered. Some candidates successfully related the solubility of compound B to its polarity.
A sample is known to contain three different amino acids. After carrying out paper chromatography using a solvent made up of propan-1-ol, water and ammonia, the following chromatogram was obtained once the spots had been developed with ninhydrin.
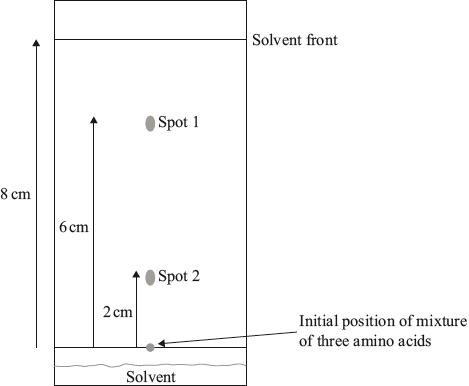
Calculate the \({R_{\text{f}}}\) values for the two spots.
Spot 1:
Spot 2:
Suggest a reason why only two spots are present.
Suggest how the chromatography experiment with the same sample could be altered in order to obtain three spots.
Markscheme
Spot 1: \({R_{\text{f}}} = 0.75\) and Spot 2: \({R_{\text{f}}} = 0.25\);
two amino acids have the same \({R_{\text{f}}}\) value;
change the polarity/make-up of the solvent / use a different solvent;
Accept two way (paper) chromatography.
Examiners report
This question was generally well answered. In part (a) some candidates reversed the answers for the spots and lost the mark. Very few linked the \({R_{\text{f}}}\) value in part (b) to the two amino acids on same spot; often the reason stated was that one amino acid did not dissolve. Part (c) was well answered. Students demonstrated a good understanding of how the nature of the solvent affects the \({R_{\text{f}}}\) value.
This question was generally well answered. In part (a) some candidates reversed the answers for the spots and lost the mark. Very few linked the \({R_{\text{f}}}\) value in part (b) to the two amino acids on same spot; often the reason stated was that one amino acid did not dissolve. Part (c) was well answered. Students demonstrated a good understanding of how the nature of the solvent affects the \({R_{\text{f}}}\) value.
This question was generally well answered. In part (a) some candidates reversed the answers for the spots and lost the mark. Very few linked the \({R_{\text{f}}}\) value in part (b) to the two amino acids on same spot; often the reason stated was that one amino acid did not dissolve. Part (c) was well answered. Students demonstrated a good understanding of how the nature of the solvent affects the \({R_{\text{f}}}\) value.
The two diagrams below show the arrangement of molecules in two different types of polyethene, labelled A and B.

Predict which type of polyethene (A or B) has the strongest intermolecular forces, highest density and greatest flexibility.
(i) Strongest intermolecular forces:
(ii) Highest density:
(iii) Greatest flexibility:
The polymer polyvinyl chloride (PVC), also known as poly(chloroethene), is hard and brittle when pure. Explain, in terms of intermolecular forces, how adding a plasticizer to PVC modifies the properties of the polymer.
Markscheme
(i) A;
(ii) A;
(iii) B;
closely packed molecules with crystalline structure;
(plasticizers) separate the PVC molecules/polymer chains / disrupt crystalline structure;
decrease/weaken intermolecular forces/intermolecular dipole-dipole interactions/van der Waals’/London Dispersion;
Do not accept mention of H-bonding
makes it (PVC) softer/more flexible/more easily moulded;
Examiners report
Many candidates were able to score three marks in (a) and most gave a good account of (b). Many, however, neglected to mention intermolecular forces, specifically requested in the question.
Many candidates were able to score three marks in (a) and most gave a good account of (b). Many, however, neglected to mention intermolecular forces, specifically requested in the question.
Amino acids are usually identified by their common names. Use section 33 of the data booklet.
State the IUPAC name for leucine.
A mixture of amino acids is separated by gel electrophoresis at pH 6.0. The amino acids are then stained with ninhydrin.
(i) On the diagram below draw the relative positions of the following amino acids at the end of the process: Val, Asp, Lys and Thr.
(ii) Suggest why glycine and isoleucine separate slightly at pH 6.5.
Determine the number of different tripeptides that can be made from twenty different amino acids.
The fibrous protein keratin has a secondary structure with a helical arrangement.
(i) State the type of interaction responsible for holding the protein in this arrangement.
(ii) Identify the functional groups responsible for these interactions.
Markscheme
2-amino-4-methylpentanoic acid
Accept 4-methyl-2-aminopentanoic acid.
i
Lys on cathode side AND Asp on anode side
Val at origin AND Thr on anode side but closer to origin than Asp
Val and Thr need not overlap.
Accept any (reasonable) size and demarcation of position so long as position relative to origin is correct.
Accept crosses for spots.
Award [1 max] for any two correct.
Award [1 max] if net direction of spots is reversed.
Award [1 max] if the four points are in the correct order but not in a straight line.
ii
different sizes/molar masses/chain lengths «so move with different speeds»
«203 =» 8000
i
hydrogen bonds
ii
carboxamide/amide/amido
OR
C=O AND N–H
Accept peptide.
Examiners report
Proteins are made of long chains of amino acids.
Explain how individual amino acids can be obtained from proteins for chromatographic separation.
A mixture of amino acids was spotted onto chromatography paper and eluted with a solvent mixture. The following spots were seen after the paper had been developed with ninhydrin.
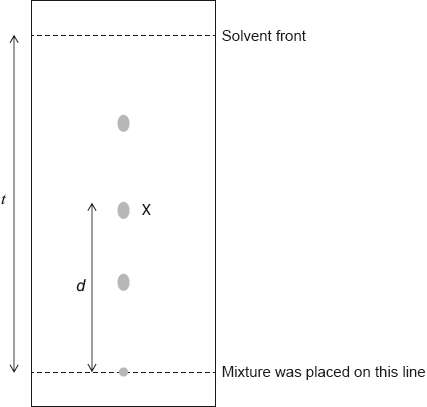
Determine the \({R_{\text{f}}}\) value of the amino acid marked as X.
One protein found in the human body is collagen. Identify its function.
Markscheme
hydrolyse/break peptide bonds;
heat/boil with (concentrated) hydrochloric acid/HCl;
Accept NaOH / enzymes.
0.50;
Accept answers in the range 0.49–0.51.
Accept d/t.
structural (protein) / connective/fibrous tissue (in tendons/ligaments/cartilage/skin) / provides elasticity/strength to skin / strengthens blood vessels;
Examiners report
A large number of candidates misinterpreted the question and described in detail how the process of chromatography would separate the amino acids. Candidates who interpreted the question correctly, often did not mention that the protein had to be heated with acid and did not refer to the peptide bonds being broken in this hydrolytic process.
The \({R_{\text{f}}}\) value was often not actually calculated but the correct formula for its calculation did score the mark.
A large number of candidates misinterpreted the question and described in detail how the process of chromatography would separate the amino acids. Candidates who interpreted the question correctly, often did not mention that the protein had to be heated with acid and did not refer to the peptide bonds being broken in this hydrolytic process.
The \({R_{\text{f}}}\) value was often not actually calculated but the correct formula for its calculation did score the mark.
A large number of candidates misinterpreted the question and described in detail how the process of chromatography would separate the amino acids. Candidates who interpreted the question correctly, often did not mention that the protein had to be heated with acid and did not refer to the peptide bonds being broken in this hydrolytic process.
The \({R_{\text{f}}}\) value was often not actually calculated but the correct formula for its calculation did score the mark.
Linolenic acid (omega-3 fatty acid) is an essential fatty acid.
List two benefits of linolenic acid to humans.
Calculate the iodine number for linolenic acid, C17H29COOH \(({M_{\text{r}}} = 278.48)\).
The condensed structural formula of linolenic acid is given in table 22 of the data booklet.
Markscheme
assists brain function;
enables normal growth/development;
involved in synthesis of prostaglandins;
reduces (risk of) heart disease/blockage of arteries;
lowers blood pressure;
reduces LDL cholesterol;
increases HDL cholesterol;
Do not accept bad/good instead of LDL/HDL.
\({{\text{n}}_{{\text{iodine}}}} = {\text{3}}{{\text{n}}_{{\text{linolenic acid}}}}\);
\({\text{iodine number}}\left( {\frac{{3 \times 100 \times 253.8}}{{278.48}}} \right) = 273{\text{ (g)}}\);
Award [2] for final correct answer.
If an alternative definition, in terms of moles/C=C bonds is given, award [1 max] for iodine number = 3.
Examiners report
The benefits of linolenic acid were reasonably well known especially its effect on lowering LDL cholesterol and many candidates scored full marks for this part of the question although in some cases, one of the marks was not achieved due to LDL not being specified. The definition of iodine number sometimes proved challenging with some confusion of mass and moles of iodine. The expected definition of the iodine number is the mass in grams of iodine reacting with 100g of oil. Overall, students did find the calculation of the iodine number challenging.
The benefits of linolenic acid were reasonably well known especially its effect on lowering LDL cholesterol and many candidates scored full marks for this part of the question although in some cases, one of the marks was not achieved due to LDL not being specified. The definition of iodine number sometimes proved challenging with some confusion of mass and moles of iodine. The expected definition of the iodine number is the mass in grams of iodine reacting with 100g of oil. Overall, students did find the calculation of the iodine number challenging.
Lipids are a diverse group of compounds found in the body.
Cholesterol is one of the most important steroids. It plays an essential role in metabolism and is the starting point for the synthesis of many important chemicals in the body.
Compare the structures and polarities of fats and phospholipids, giving one similarity and one difference in structure and one difference in polarity.
Similarity in structure:
Difference in structure:
Difference in polarity:
Vitamin D is produced from cholesterol. The structures of both molecules are given in table 21 of the data booklet. Outline one structural difference between the molecules.
Distinguish between HDL and LDL cholesterol in terms of their composition and their effect on health.
Composition:
One effect on health:
Markscheme
Similarity in structure:
both are (tri)esters / both made from glycerol/propane-1,2,3-triol/HOCH2CH(OH)CH2OH;
Difference in structure:
phospholipids have phosphate group/phosphorus and fats are triglycerides/made from three fatty/carboxylic acids / one fatty/carboxylic acid (in fat) replaced by phosphate in phospholipid;
Difference in polarity:
phospholipids are more polar / phospholipids have hydrophilic (heads/section/part/end) / fats are less polar/non-polar / fats are hydrophobic ;
(two) more carbon−carbon double bonds/alkenyl groups in vitamin D;
Accept alkene for alkenyl.
extra hexagon/6-membered ring in cholesterol / more fused rings in cholesterol / four fused rings in cholesterol and two fused rings in vitamin D;
Accept “(some) conjugation in vitamin D / (some) alternating C=C and C−C bonds in vitamin D”.
Accept “cholesterol has a steroid backbone/structure but vitamin D does not”.
Composition:
HDL has more protein and less cholesterol/fat/lipid (and vice-versa);
Accept “HDL has more protein and LDL has more cholesterol (and vice-versa)”.
Accept “HDL has higher phospholipid content compared to LDL (and vice-versa)”.
Accept “HDL particles are smaller than LDL particles (and vice-versa)” but do not penalize if “molecules” are used instead of “particles”.
One effect on health:
cardiovascular problems/increased risk of heart disease/obesity/atherosclerosis/blocked arteries from high ratio of LDL to HDL;
Accept “from (high) LDL” instead of “from high ratio of LDL to HDL”.
Accept “can result in a heart attack/stroke from high ratio of LDL to HDL”.
Accept “large amounts of HDL in blood correlate with good health / OWTTE”.
Reference must be made to LDL or HDL.
Examiners report
About half the candidates seemed familiar with the structures of fats and phospholipids, but only a few gave the detailed answers expected by the markscheme. Some of the answers were far too general such as stating “both contain C, H and O” for the similarity in structure.
About a third of the candidates scored the mark by stating an accurate structural difference between vitamin D and cholesterol. Some answers were not specific enough about the numbers and types of rings.
Only a few candidates were able to distinguish between the composition of HDL and LDL cholesterol, but the majority of candidates understood their effects on health well and gained the second mark. There was a comment on a G2 form that this question went beyond the syllabus, however, the question is covered in part B.4.2 (“outline the difference between HDL and LDL cholesterol”) and has also appeared in a past paper.
Draw the structure of a 2-amino acid.
(i) Using Table 19 of the Data Booklet, draw the structure of the two dipeptides formed by the reaction of glycine with valine.
(ii) State the other product of the reaction in (i).
Explain how a given protein can be broken down into its constituent amino acids and how these can be identified by electrophoresis.
Markscheme
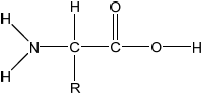 ;
;
Allow condensed structural formula (eg, R-CH(NH2)COOH) or structure of a specific 2-amino acid (eg, Ala etc.).
(i) 
Accept condensed structural formulas.
Accept C3H7 for CH3CHCH3.
(ii) \({{\text{H}}_{\text{2}}}{\text{O}}\)/water;
add hydrochloric acid/HCl to separate individual amino acids;
Accept strong acid/concentrated H+ or restriction enzymes but not sulfuric acid/H2SO4.
Award [4 max] for any four of:
mixture/amino acids spotted/placed on gel/PAGE/polyacrylamide/paper;
gel placed in buffer/solution of known pH;
voltage/potential difference applied;
Accept “applied electric field / positive and negative electrodes connected / anode and cathode connected” but not current.
amino acids move differently depending on size and charge/isoelectric point/pH of buffer / amino acids move to oppositely charged electrodes;
Accept any suitable diagram.
develop with ninhydrin/(organic) dye / detect by UV (light)/staining/fluorescence;
Accept any suitable development method.
measure distances moved / compare with known samples / measure isoelectric points;
Examiners report
In (a) most candidates drew either the general formula of a 2-amino acid or drew the structure of a specific 2-amino acid.
In (b) (i) the structures of the two dipeptides formed by the reaction of glycine with valine were usually correctly represented and water was identified by almost all.
In (c) electrophoresis was well understood as a biochemical analytical technique. However some candidates did not read the question carefully and dropped the first mark for not stating that hydrochloric acid needed to be added to separate the individual amino acids.
Although people may consume a large amount of food, they may still not consume sufficient nutrients.
Describe one similarity and one difference between the structure of a saturated and an unsaturated fat.
Similarity:
Difference:
Markscheme
Similarity:
both are (tri)esters / both made from glycerol/propane-1,2,3-triol/HOCH2CH(OH)CH2OH / both are triglycerides;
Difference:
unsaturated fats have C=C/carbon−carbon double bond / saturated fats have no C=C/carbon−carbon double bonds;
Examiners report
Many candidates are still forgetting to specify that the difference is in carbon-carbon double bonds. This is important as both fats contain carbon-oxygen double bonds.
A chemical reaction occurs when a phospholipid is heated with excess sodium hydroxide.
Glycerol is one product of the reaction. Identify the two other organic products.
Identify the type of reaction which occurs.
Markscheme
C17H31COONa
[(CH3)3NCH2CH2OH]OH
Accept “NaC17H31COO”.
Accept “(CH3)3N+CH2CH2OH OR [(CH3)3NCH2CH2OH]+” if positive charge is shown.
Accept suitable names (eg, sodium linoleate, choline hydroxide etc.) OR correct molecular formulas.
[2 marks]
hydrolysis
Accept “nucleophilic substitution/displacement / SN/SN2 /saponification”.
Do not accept “acid hydrolysis”.
[1 mark]
Examiners report
Lipids and carbohydrates contain the same elements but have different properties.
List the building blocks of triglycerides and carbohydrates.
The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid, C18H32O2, but not by the trisaccharide, raffinose, C18H32O16, containing the same number of carbon atoms.
Explain why raffinose is far more water soluble than linoleic acid.
Solid fat triglycerides can also clog kitchen sink drains.
Explain how sodium hydroxide unblocks the drain.
The amount of proteins, fats and carbohydrates determine the energy content of foods.
Explain why linoleic acid, C18H32O2, is a more efficient energy storage molecule than raffinose, C18H32O16.
Markscheme
Triglycerides:
organic acid/fatty acid and glycerol/propane-1,2,3-triol
AND
Carbohydrates:
monosaccharides
Accept simple sugars.
[1 mark]
«water/aqueous solubility depends on forming many» H-bonds with water
raffinose has many hydroxyl/O–H/oxygen atoms/O «and forms many H-bonds» AND linoleic acid has few/one hydroxyl/O–H/oxygen atom/O/carboxyl group/ COOH/is largely non-polar «and cannot form many H-bonds»
Accept statement which implies comparison.
[2 marks]
«base» hydrolysis/saponification
OR
«produces glycerol and» soap/salt of the «fatty» acid
«products are» water soluble «and drain away»
Accept condensed formulas.
Accept non-balanced equation.
Accept “RCOONa”.
[2 marks]
linoleic acid/C18H32O2 combustion/oxidation more exothermic «per mol»
linoleic acid/C18H32O2 has lower proportion/number of O atoms
OR
linoleic acid/C18H32O2 is less oxidized
Accept converse arguments.
[2 marks]
Examiners report
Peptidase enzyme in the digestive system hydrolyses peptide bonds.
A tripeptide Ala-Asp-Lys was hydrolysed and electrophoresis of the mixture of the amino acids was carried out at a pH of 6.0. Refer to section 33 of the data booklet.
Identify the type of metabolic process that occurs in the hydrolysis of the peptide during digestion.
Identify the name of the amino acid that does not move under the influence of the applied voltage.
Deduce, giving a reason, which amino acid will develop closest to the negative electrode.
The breakdown of a dipeptide in the presence of peptidase was investigated between 18 °C and 43 °C. The results are shown below.
Comment on the rate of reaction at temperature X in terms of the enzyme’s active site.
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
Pollution from heavy metal ions has become a health concern.
Outline how the presence of heavy metal ions decreases the action of enzymes.
Outline how lead ions could be removed from an individual suffering from lead poisoning.
Markscheme
catabolism/catabolic
[1 mark]
alanine
Do not accept ala.
[1 mark]
Lys/lysine
pH «buffer» < pI «Lys»
OR
buffer more acidic than Lys «at isoelectric point»
OR
«Lys» exists as
OR
«Lys» charged positively/has +1/1+ «overall» charge «and moves to negative electrode»
Do not apply ECF from M1.
Accept converse argument.
Do not accept just “has H3N+ group” for M2 (as H3N+ is also present in zwitterion).
Do not penalize if COOH is given in the structure of lysine at pH 6 instead of COO–.
[2 marks]
highest frequency of successful collisions between active site and substrate
OR
highest frequency of collisions between active site and substrate with sufficient energy/\(E \geqslant {E_{\text{a}}}\) AND correct orientation/conformation
OR
optimal shape/conformation of the active site «that matches the substrate»
OR
best ability of the active site to bind «to the substrate»
Accept “number of collisions per unit time” for “frequency”.
Do not accept “all active sites are occupied”.
[1 mark]
ascorbic acid/vitamin C
[1 mark]
react/bind/chelate with enzyme
OR
disrupt ionic salt bridges
OR
affect shape of tertiary/quaternary structures
OR
precipitate enzymes
OR
break/disrupt disulfide bridges/bonds
Do not accept “changes shape of active site” by itself.
[1 mark]
«use of» host-guest chemistry
OR
chelation «therapy»
Accept specific medication/chelating agent such as EDTA, CaNa2 EDTA, succimer, D-penicillamine, dimercaprol.
[1 mark]
Examiners report
The structures of the amino acids cysteine, glutamine and lysine are given in section 33 of the data booklet.
Deduce the structural formula of the dipeptide Cys-Lys.
Identify the type of bond between two cysteine residues in the tertiary structure of a protein.
Deduce the structural formula of the predominant form of cysteine at pH 1.0.
A mixture of the three amino acids, cysteine, glutamine and lysine, was placed in the centre of a square plate covered in polyacrylamide gel. The gel was saturated with a buffer solution of pH 6.0. Electrodes were connected to opposite sides of the gel and a potential difference was applied.
Sketch lines on the diagram to show the relative positions of the three amino acids after electrophoresis.
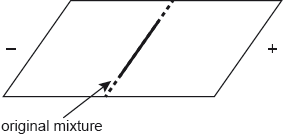
Markscheme
correct order
amide link
Accept CO–NH but not CO–HN for amide link.
Penalize incorrect bond linkages or missing hydrogens once only in 7 (a) and 7 (c).
[2 marks]
covalent
Accept “S-S/disulfide”.
[1 mark]
Penalize incorrect bond linkages or missing hydrogens once only in 7 (a) and 7 (c).
[1 mark]
Cys and Gln move to positive electrode AND Lys to negative electrode
Cys further to positive electrode than Gln
Do not penalize if lines are omitted or if different markings are given (eg, spots etc.), as long as relative positions are correctly indicated.
Accept Gln on original position indicated.
Award [1 max] for reverse order of amino acids.
[2 marks]
Examiners report
Dehydroepiandrosterone (DHEA) is a substance banned under the World Anti-Doping Code.
Steroid abuse has certain health hazards, some general, some specific to males and some specific to females. Identify one health hazard in each category.
General Hazard:
Male Hazard:
Female Hazard:
(i) State the name of the functional group circled in the DHEA molecule shown below.
(ii) Identify the characteristic of this structure that classifies it as a steroid.
The production of banned steroids has ethical implications. Suggest a reason why steroid research might be supported.
Markscheme
General hazards:
acne
OR
weight gain
OR
liver/kidney damage
OR
stunted growth
OR
disruption of puberty
OR
increased aggressiveness
OR
increased risk of heart disease/atherosclerosis/heart attacks/strokes√
General hazards:
Accept heart problems.
Male hazards:
feminization/breast «tissue» development
OR
shrinking of the testes/testicles
OR
reduction in sperm production
OR
impotence
Male hazards:
Accept baldness.
Female hazards:
decreased breast development
OR
masculinisation
OR
infertility/abnormal menstrual cycles
OR
birth defects/altered fetus development
(i)
alkenyl/ethanylylidene
(ii)
four-ring «steroidal» backbone
OR
fused ring structure
OR
three 6-membered rings AND a 5-membered ring
Award [1] for a sketch of the steroidal backbone.
medical uses of steroids «under physician supervision»
OR
detection of banned substances can be improved
Accept any specific medical use.
Accept answers such as “their effects «either positive or negative» are better understood”.
Examiners report
Amino acids, shown in section 33 of the data booklet, can be combined to form polypeptides and proteins.
Deduce the structures of the most abundant form of glycine in three buffer solutions at pH 1.0, 6.0 and 11.0.
A tripeptide, X, containing leucine (Leu), lysine (Lys) and glutamic acid (Glu) is hydrolysed and separated by gel electrophoresis in a buffer solution with a pH of 6.0.
(i) Predict the result of the electrophoresis by labeling the three spots below with the names of the amino acids.
(ii) Deduce the number of tripeptides that could be formed by using the three amino acids of tripeptide X.
Markscheme
Charges must be shown on the correct atoms in each structure for mark. Penalize repeated mistakes once.
Although question asks specifically for structures, accept condensed structural formulas, but charges must be given.
(i)
Award [2] for correct order.
Award [1 max] for Leu in centre if order is incorrect.
(ii)
6
Accept 27.
Examiners report
Carbohydrates are energy-rich molecules which can be synthesized in some plant cells from inorganic compounds.
State the raw materials and source of energy used in the process described above.
The structures of two molecules, X and Y, are shown below.
(i) Justify why both these molecules are carbohydrates.
(ii) Distinguish between these molecules in terms of their functional groups.
Amylose is an unbranched polysaccharide composed of repeating units of glucose.
(i) Draw the structure of the repeating unit of amylose. Use section 34 of the data booklet.
(ii) Amylose is a major component of starch. Corn starch can be used to make replacements for plastics derived from oil, especially for packaging. Discuss one potential advantage and one disadvantage of this use of starch.
Markscheme
CO2 AND H2O AND sun
Accept names.
Accept “sunlight/light/photons” instead of “sun”.
i
both have formula Cx(H2O)y
OR
both contain several OH/hydroxyl «groups» AND a C=O/carbonyl «group»
Accept “both have the formula CnH2nOn /empirical formula CH2O” but do not accept “both have same molecular formula/have formula C3H6O3”.
Accept “aldehyde or ketone” for “carbonyl”.
ii
Accept “alkyl” for “R”.
Accept “X: aldose/aldehyde AND Y: ketose/ketone”.
Accept “CO” for “C=O”.
i
continuation bonds AND open O on either but not both ends
Brackets are not necessary for the mark.
Do not accept β-isomer.
Mark may be awarded if a polymer is shown but with the repeating unit clearly identified.
3-D representation is not required.
ii
Advantage:
Any one of:
biodegradable / break down naturally/by bacteria
Do not accept just “decompose easily”.
compostable
does not contribute to land-fill
renewable/sustainable resource
starch grains swell AND help break up plastic
lower greenhouse gas emissions
uses less fossil fuels than traditional plastics
less energy needed for production
Disadvantage:
Any one of:
land use «affects biodiversity/loss of habitat»
growing corn for plastics instead of food
«starch» breakdown can increase acidity of soil/compost
«starch» breakdown can produce methane «especially when buried»
sensitive to moisture/bacteria/acidic foods
«bioplastics sometimes» degrade quickly/before end of use
cannot be reused
poor mechanical strength
eutrophication
increased use of fertilizers/pesticides/phosphorus/nitrogen «has negative environmental effects»
Ignore any reference to cost.
Accept “prone to site explosions/fires” or “low heat resistance” for disadvantage.
Only award [1 max] if the same example is used for the advantage and disadvantage.
Examiners report
Monosaccharides can combine to form disaccharides and polysaccharides.
Identify the functional groups which are present in only one structure of glucose.
Sucrose is a disaccharide formed from \(\alpha \)-glucose and β-fructose.
Deduce the structural formula of sucrose.
Starch is a constituent of many plastics. Suggest one reason for including starch in plastics.
Suggest one of the challenges scientists face when scaling up the synthesis of a new compound.
Markscheme
Only in straight chain form:
carbonyl
OR
aldehyde
Only in ring structure:
hemiacetal
Accept functional group abbreviations (eg, CHO etc.).
Accept “ether”.
[2 marks]
correct link between the two monosaccharides
Correct 1,4 beta link AND all bonds on the 2 carbons in the link required for mark.
Ignore any errors in the rest of the structure.
Penalize extra atoms on carbons in link.
[1 mark]
plastic «more» biodegradable/degrades into nontoxic products
OR
plastic can be produced using green technology/renewable resource
OR
reduces fossil fuel use/petrochemicals
OR
easily plasticized
OR
used to form thermoplasts
[1 mark]
minimize «negative» impact on environment
OR
minimize waste produced
OR
consider atom economy
OR
efficiency of synthetic process
OR
problems of side reactions/lower yields
OR
control temperature «inside large reactors»
OR
availability of starting/raw materials
OR
minimize energy costs
OR
value for money/cost effectiveness/cost of production
[1 mark]