
HL Paper 3
Insulin was the first protein to be sequenced. It was determined that the end of one chain had the primary structure Phe–Val–Asn–Gln.
Describe how DNA determines the primary structure of a protein such as insulin.
DNA is a biopolymer made up of nucleotides. List two components of a nucleotide.
DNA is a complex molecule.
Outline how its structure allows it to be negatively charged in the body.
Deduce the nucleotide sequence of a complementary strand of a fragment of DNA with the nucleotide sequence –GACGGATCA–.
A hemoglobin-oxygen saturation curve does not follow the same model as enzyme-substrate reactions.
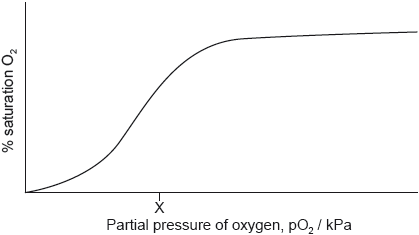
Explain the shape of the curve from 0 to X kPa.
Explain why carbon monoxide is toxic to humans.
The heme groups in cytochromes contain iron ions that are involved in the reduction of molecular oxygen.
State the half-equation for the reduction of molecular oxygen to water in acidic conditions.
Outline the change in oxidation state of the iron ions in heme groups that occurs when molecular oxygen is converted to water.
Anthocyanins are naturally occurring plant pigments. Depending on the solution pH, they can exist as quinoidal bases or flavylium cations as shown in section 35 of the data booklet.
Outline why anthocyanins are coloured.
Explain why the blue colour of a quinoidal base changes to the red colour of a flavylium cation as pH decreases.
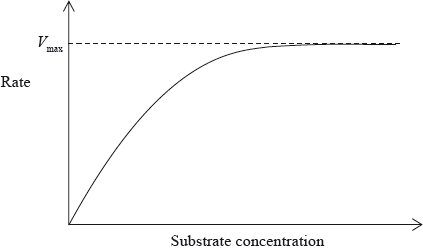
The following graph shows the effect of substrate concentration on the rate of an enzyme-catalysed reaction.
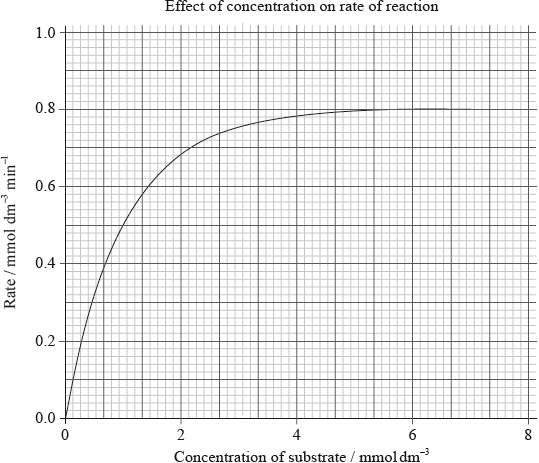
Explain the relationship between enzyme activity and concentration of the substrate.
Determine the Michaelis constant \({K_{\text{m}}}\) from the graph.
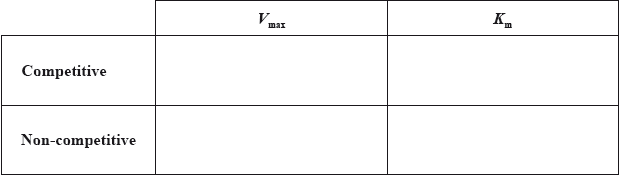
Describe why competitive inhibition may take place.
Explain the effect of competitive inhibition on \({V_{{\text{max}}}}\) and \({K_{\text{m}}}\).
On the graph of effect of concentration on rate of reaction on page 10, sketch the expected curve for non-competitive inhibition.
DNA is the genetic material that individuals inherit from their parents. Genetic information is stored in chromosomes which are very long strands of DNA.
Describe the structure of a nucleotide of DNA.
Outline how nucleotides are linked together to form polynucleotides.
Outline the process of hydrogenating fats and name one catalyst for the process.
Anthocyanins and carotenes are both coloured substances found in many foods.
Explain, in terms of their molecular structure, why these compounds are coloured.
Identify one other coloured compound commonly found in uncooked foods.
Stereochemistry is the study of the spatial arrangement of atoms in molecules. A molecule containing a chiral carbon atom exists as two enantiomers. Three different conventions can be used for naming purposes.
Use the CORN rule to determine whether the structure of 2-aminopropanoic acid (alanine) represents the D or L form. Justify your answer.
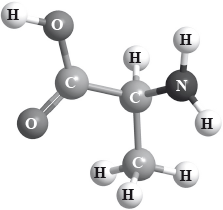
State the (d) or the (l) convention.
A food product is often judged simply by its colour. Natural pigments that give rise to food colour include anthocyanins, carotenes, chlorophyll and heme. The structures of these pigments are shown in Table 22 of the Data Booklet.
Explain why these natural pigments are coloured.
Deduce from their structures whether anthocyanins and carotenes are water-soluble or fat-soluble.
Anthocyanins:
Carotenes:
State and explain how the rate of an enzyme-catalysed reaction is related to the substrate concentration.
When an inhibitor is added it decreases the rate of an enzyme-catalysed reaction. State the effect that competitive and non-competitive inhibitors have on the value of \({V_{\max }}\). Explain this in terms of where the inhibitor binds to the enzyme.
Competitive inhibitor:
Non-competitive inhibitor:
(i) Sketch a graph to show the effect that a change in pH will have on the rate of an enzyme-catalysed reaction.
(ii) Explain why changing the pH affects the catalytic ability of enzymes.
Hemoglobin contains a heme group with an iron(II) ion.
Outline how the oxygen saturation of hemoglobin is affected by changes in the blood plasma.
Explain why foetal hemoglobin has a greater affinity for oxygen.
An inhibitor reduces the rate, V, of an enzyme-catalysed reaction.
Explain with reference to the binding site on the enzyme how a non-competitive inhibitor lowers the value of Vmax.
Outline the significance of the value of the Michaelis constant, Km.
Polymers of glucose include starch and cellulose.
Outline why cellulose fibres are strong.
DNA and RNA both contain a pentose sugar.
State the names of the sugars in each nucleic acid and outline how their chemical structures differ.
State one other structural difference between DNA and RNA.
The kinetics of an enzyme-catalysed reaction are studied in the absence and presence of an inhibitor. The graph represents the initial rate as a function of substrate concentration.
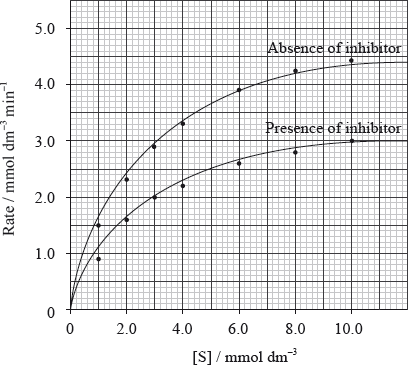
Identify the type of inhibition shown in the graph.
Determine \({V_{\max }}\) and \({K_{\text{m}}}\) in the absence of the inhibitor and in the presence of the inhibitor.
Absence of inhibitor:
Presence of inhibitor:
Outline the relationship between \({K_{\text{m}}}\) and enzyme activity.
A large proportion of the food we eat provides energy through the process of respiration. Carbohydrates and triglycerides are the food groups mainly responsible for providing this energy.
Compare the behaviour of enzymes and inorganic catalysts, including reference to the mechanism of enzyme action and the ways in which this can be inhibited.
The structure of DNA (deoxyribonucleic acid) has been studied in many different ways.
State the name of the component of DNA responsible for the migration of its fragments to the positive electrode in gel electrophoresis.
In 2010, scientists claimed that they had discovered a species of bacteria capable of incorporating arsenic in place of phosphorus into the bacterial DNA. This claim has since proved controversial. Suggest one technique or evidence that might help support the claim.
The stereochemistry of molecules affects the way they interact with taste and smell receptors in the body.
The stereochemistry of carbohydrates and amino acids is usually indicated by the D/L convention.
Alanine has the formula \({\text{[}}{{\text{H}}_{\text{2}}}{\text{N}}–{\text{CH(C}}{{\text{H}}_{\text{3}}}{\text{)}}–{\text{COOH]}}\). Deduce the structure of D-alanine and complete its structure below.
State which convention is usually employed to indicate the stereochemistry of molecules other than carbohydrates and amino acids.
Based on the structure given in part (b) (i) comment on the statement “D-alanine is a +(d) compound”.
Amino acids are the building blocks of proteins.
Draw the structures of the main form of glycine in buffer solutions of pH 1.0 and 6.0.
The pKa of glycine is 2.34.
Calculate the pH of a buffer system with a concentration of 1.25 × 10−3 mol dm−3 carbonic acid and 2.50 × 10−2 mol dm−3 sodium hydrogen carbonate. Use section 1 of the data booklet.
pKa (carbonic acid) = 6.36
Sketch the wedge and dash (3-D) representations of alanine enantiomers.
UV-Vis spectroscopy can be used to determine the unknown concentration of a substance in a solution.
Calculate the concentration of an unknown sample of pepsin with an absorbance of 0.725 using section 1 of the data booklet.
Cell length = 1.00 cm
Molar absorptivity (extinction coefficient) of the sample = 49650 dm3 cm−1 mol−1
A different series of pepsin samples is used to develop a calibration curve.
Estimate the concentration of an unknown sample of pepsin with an absorbance of 0.30 from the graph.
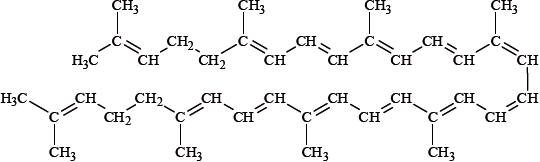
Lycopene, whose structure is shown below, is a carotenoid and is responsible for the red colour in tomatoes. When bromine is slowly added to some tomato juice, the colour of the juice gradually changes from red to yellow. Explain this colour change in terms of changes in bonding in lycopene.
Enzymes are protein molecules that catalyse specific biochemical reactions. The phosphorylation of glucose is the first step of glycolysis (the oxidation of glucose) and is catalysed by the enzyme hexokinase.
Explain how enzymes, such as hexokinase, are able to catalyse reactions.
State and explain the effect of increasing the temperature from 20 °C to 60 °C on an enzyme-catalysed reaction.
Explain how the structure of vitamin A is important to vision using section 35 of the data booklet.
Vitamins can be water-soluble or fat-soluble.
Retinal is the key molecule involved in vision. Explain the roles of cis and trans-retinal in vision and how the isomers are formed in the visual cycle.
The nucleic acids, RNA and DNA, are polymers which are formed from nucleotides. Distinguish between the structures of RNA and DNA.
In aerobic respiration, the metabolism of glucose takes place using the processes of oxidation and reduction.
Identify the molecule that undergoes oxidation and state the half-equation for the process.
Identify the molecule that undergoes reduction and state the half-equation for the process.
Enzymes are biological catalysts. Catalases are highly efficient enzymes found in cells. Each catalase molecule can decompose millions of hydrogen peroxide molecules per second.
Metal-based inorganic catalysts are also common. In 2009, at Cardiff University in Wales, a new catalyst was developed by Hutchings and co-workers using gold–palladium nanoparticles in the direct synthesis of hydrogen peroxide.
Enzymes are affected by inhibitors which can be either competitive or non-competitive.
Describe the characteristics of an enzyme (such as catalase).
State how inhibitors affect the initial rate of reaction of an enzyme with its substrate.
Explain the action of competitive and non-competitive inhibitors on enzymes in terms of where the inhibitor binds to the enzyme.
Competitive inhibitors:
Non-competitive inhibitors:
State how inhibitors affect the values of \({V_{{\text{max}}}}\) and the Michaelis constant, \({K_{\text{m}}}\), by completing the table below.
Amino acids are usually identified by their common names. Use section 33 of the data booklet.
Amino acids act as buffers in solution. In aspartic acid, the side chain (R group) carboxyl has pKa = 4.0. Determine the percentage of the side chain carboxyl that will be ionized (–COO–) in a solution of aspartic acid with pH = 3.0. Use section 1 of the data booklet.
The enzyme hexokinase catalyses one of the initial reactions between glucose and adenosine triphosphate (ATP) during the process of glycolysis.
The graph below shows how the rate of this enzyme-catalysed reaction changes as the glucose concentration is increased.
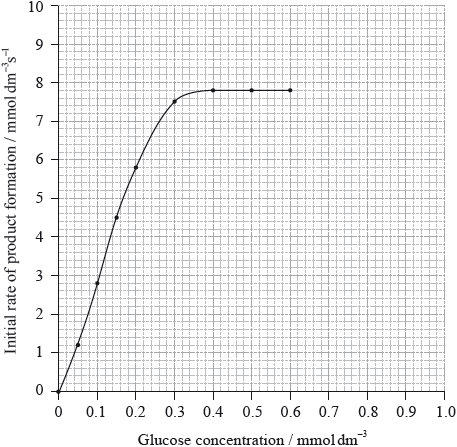
From the graph, determine \({V_{{\text{max}}}}\) and the Michaelis constant, \({K_{\text{m}}}\).
\({V_{{\text{max}}}}\):
\({K_{\text{m}}}\):
Explain why a low value of \({K_{\text{m}}}\) is significant.
State and explain the effect of a competitive inhibitor on the value of \){K_{\text{m}}}\).
Calculate the number of carbon-carbon double bonds in linolenic acid, \({{\text{C}}_{{\text{18}}}}{{\text{H}}_{{\text{30}}}}{{\text{O}}_{\text{2}}}\), given that 7.7 g of iodine, \({{\text{I}}_{\text{2}}}\), react with 2.8 g of linolenic acid.
Vitamins are organic micronutrients essential for good health. The structures of vitamins A, C and D are given in Table 21 of the Data Booklet.
Vitamin D is the only vitamin that can be synthesized in the body, by the action of sunlight on the skin.
Identify by name two functional groups that are common to all three of these vitamins.
Only one of these three vitamins is soluble in water.
Identify this vitamin.
Explain why this vitamin is soluble in water.
State one effect of vitamin D deficiency.
Suggest why vitamin D deficiency diseases are becoming increasingly common in young people.
Hemoglobin is often described as a carrier of diatomic oxygen.
Describe the structure of hemoglobin.
Outline the role of hemoglobin in transporting diatomic oxygen.
The following products result from the hydrolysis of a triglyceride.
\({{\text{C}}_{{\text{19}}}}{{\text{H}}_{{\text{31}}}}{\text{COOH}}\) \({{\text{C}}_{{\text{13}}}}{{\text{H}}_{{\text{27}}}}{\text{COOH}}\) \({{\text{C}}_{{\text{15}}}}{{\text{H}}_{{\text{29}}}}{\text{COOH}}\)
Draw a possible structure for the triglyceride.
State the other reactant and one essential condition that would favour this hydrolysis reaction in the body.
Identify which product is polyunsaturated, and outline why foods containing this type of fatty acid are important for health.
Enzymes are catalysts that increase the rate of all biochemical reactions, including those involved in respiration.
Cytochrome oxidase is a complex enzyme that catalyses the reduction of oxygen in the final stage of aerobic respiration. This enzyme is inhibited both by nitrogen(II) oxide, NO, and separately by cyanide ions, \({\text{C}}{{\text{N}}^ - }\). It has been suggested that NO acts competitively while \({\text{C}}{{\text{N}}^ - }\) acts non-competitively in inhibiting the enzyme. Experiments were carried out to test this hypothesis.
The graph below shows the effect of substrate concentration on the rate of the reaction in the absence of an inhibitor. Draw and label the results of the two experiments showing how the rate of the reaction changes in the presence of NO and in the presence of \({\text{C}}{{\text{N}}^ - }\), if the hypothesis is correct.
Suggest a reason why it is more likely that NO, rather than \({\text{C}}{{\text{N}}^ - }\), acts competitively.
The reducing agent in the cytochrome oxidase reaction is a species that can be denoted as \({\text{X}}{{\text{H}}_{\text{2}}}\) in the reduced form. Using this notation, deduce an equation for the reaction of \({\text{X}}{{\text{H}}_{\text{2}}}\) and \({{\text{O}}_{\text{2}}}\), and outline, using oxidation numbers, why it is a redox reaction.
Cholesterol belongs to a class of substances named lipids.
Describe one negative effect of a high concentration of LDL cholesterol in blood.
Nucleic acids are natural polymers with exceptionally large relative molecular masses, made up of nucleotides. All cells in the human body, with the exception of red blood cells, contain deoxyribonucleic acid (DNA).
James Watson, Francis Crick and Maurice Wilkins were awarded the 1962 Nobel Prize in Physiology or Medicine “for their discoveries concerning the molecular structure of nucleic acids and its significance for information transfer in living material”.
(i) Explain how the two helices are linked in the structure of DNA.
(ii) Describe the role of DNA in the storage of genetic information. The details of protein synthesis are not required.
The colour of olive oil is due to pigments such as chlorophyll, pheophytin and carotenoids.
The absorption spectrum of one form of pheophytin is shown below.
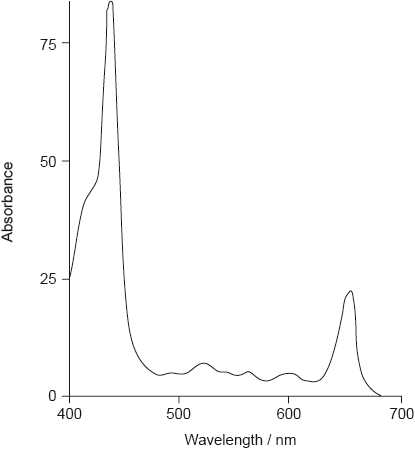
State the structural feature of a pheophytin molecule which allows it to absorb visible light.
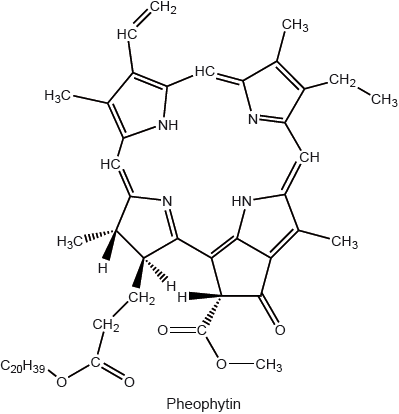
Carotenoids may lose their colour and develop off odours when they are oxidized.
Identify, using table 22 of the data booklet, the structural feature that makes carotenoids susceptible to oxidation.
List two factors which increase the rate of oxidation of carotenoids.
Deduce, giving a reason, whether carotenoids are water-soluble or fat-soluble.
Deoxyribonucleic acid (DNA) is the genetic material that an individual inherits from both parents. DNA consists of nucleotides bonded together.
Outline the essential features of the structure of a section of one strand of DNA.
Olive oil is a complex mixture of triglycerides, some of which are derived from oleic acid.
Explain why oleic acid, cis-9-octadecenoic acid, has a lower melting point than its trans isomer, elaidic acid.
Discuss two effects on health of consuming trans fatty acids such as elaidic acid.
Enzymes are proteins which play an important role in the biochemical processes occurring in the body.
The graph below shows how the rate of an enzyme-catalysed reaction changes as the substrate concentration is increased.
State the major function of enzymes in the human body.
Describe the mechanism of enzyme action in terms of structure.
Use the graph to determine \({V_{\max }}\) and the Michaelis constant, \({K_{\text{m}}}\).
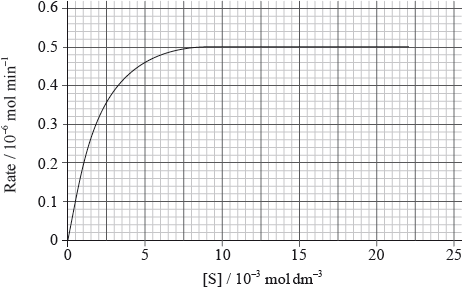
\({V_{\max }}\):
\({K_{\text{m}}}\):
Draw a line on the graph to represent the effect of adding a competitive inhibitor.
State and explain the effects of heavy-metal ions and temperature increases on enzyme activity.
Many lipids are found in the human body. One type of lipid is a triglyceride
To measure the degree of unsaturation of a lipid the iodine number can be calculated.
Calculate the iodine number of linoleic acid.
\[\begin{array}{*{20}{l}} {{\text{C}}{{\text{H}}_{\text{3}}}{{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}}_{\text{4}}}{{{\text{(CH=CHC}}{{\text{H}}_{\text{2}}}{\text{)}}}_{\text{2}}}{{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}}_{\text{6}}}{\text{COOH}}}&{{M_{\text{r}}} = 280.4} \end{array}\]
Proteins are polymers of 2-amino acids. The structures of the common amino acids are given in Table 19 of the Data Booklet. This question refers to the two amino acids alanine and cysteine.
State the structural formula of cysteine as a zwitterion.
With reference to the isoelectric points of alanine and cysteine, describe with a reason what pH value would be suitable to use in an electrophoresis experiment designed to separate these two amino acids in solution.
Cysteine is responsible for a specific type of intra-molecular bonding within a protein molecule. State the name of this type of interaction and outline how it is different from other interactions responsible for the tertiary structure.
Compare the structures of the natural pigments, chlorophyll and heme B using Table 22 of the Data Booklet.
Retinal, one of the many forms of vitamin A, reacts with opsin to produce rhodopsin. Refer to section 35 of the data booklet for one structure of vitamin A.
Identify the structural feature which enables rhodopsin to absorb visible light.
Outline the change that occurs in the retinal residue during the absorption of visible light.
Nucleic acids, which are polynucleotides present in cells, transmit essential genetic information.
Explain the double helical structure of DNA, including the importance of hydrogen bonding.
Limonene is a chiral molecule. The enantiomer found in oranges is shown below.
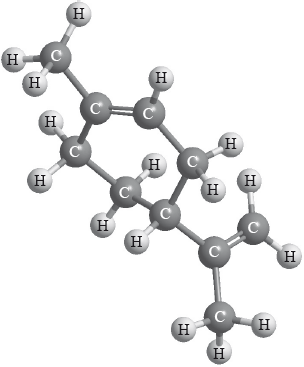
Identify the chiral carbon atom in the structure above with an asterisk, *.
Stearic acid, oleic acid and linolenic acid are all fatty acids that contain 18 carbon atoms. Their structures are given in Table 22 of the Data Booklet.
Partial hydrogenation of linolenic acid may lead to a product known as a trans fatty acid.
Explain which acid has the highest melting point.
State the equation for the complete hydrogenation of linolenic acid. Describe the conditions used for this reaction.
Explain the meaning of the term trans.
Draw the structure of a possible trans fatty acid product.
The structures of the amino acids cysteine, glutamine and lysine are given in section 33 of the data booklet.
An aqueous buffer solution contains both the zwitterion and the anionic forms of alanine. Draw the zwitterion of alanine.
Calculate the pH of a buffer solution which contains 0.700 mol dm–3 of the zwitterion and 0.500 mol dm–3 of the anionic form of alanine.
Alanine pKa = 9.87.
Strawberries have a bright red colour and a distinctive smell.
Ripe strawberries contain the flavylium cation, an anthocyanin. By referring to table 22 of the data booklet, explain why ripe strawberries are red.
Outline the difference in solubility in water between anthocyanins and carotenes, by referring to their structures in table 22 of the data booklet.
Outline another convention used for specifying a molecule’s spatial configuration and its relationship with the (\( + \)) and (\( - \)) notation (previously referred to as \(d\) and \(l\)).
The most important components of nucleotides are the nitrogeneous bases, which consist of pyrimidines and purines.
Thymine (T), whose structure is given in Table 21 of the Data Booklet, is a pyrimidine.
Describe how thymine forms part of a nucleotide in deoxyribonucleic acid (DNA).
Adenine, A, whose structure is also given in Table 21 of the Data Booklet, is a purine found in DNA.
Draw the structure of the organic product formed from the condensation reaction of adenine with the sugar D-ribose (whose structure is given below) and identify the other product.
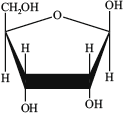
Structure of organic product:
Other product:
(i) Adenine (A), guanine (G), cytosine (C) and thymine (T) result in the double helix structure of DNA. Using the structures of adenine and thymine, draw a diagram to explain how thymine is able to play a role in forming a double helix.
(ii) Compare the bonding between cytosine and guanine with the bonding between adenine and thymine.
The graph below shows the effect of substrate concentration on the rate of an enzyme-catalysed reaction.
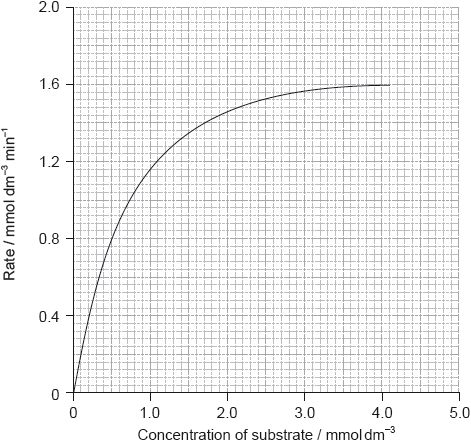
Outline the relationship between enzyme activity and concentration of the substrate.
Explain how competitive inhibition in an enzyme-catalysed reaction takes place.
Sketch, on the graph on page 13, a curve which shows competitive inhibition occurring in this reaction.
Silver ions bond with sulfur atoms in an enzyme and change its tertiary structure and activity. Predict the effect of silver ions on the values of \({V_{{\text{max}}}}\) and \({K_{\text{m}}}\) of this enzyme.
\({V_{{\text{max}}}}\):
Km :
Describe how genetic information is encoded within the double helical structure of DNA.
State the name of the bond between complementary base pairs of DNA.
Explain the bonding between base pairs by drawing the complementary base next to thymine below and by showing the bonds that hold the pairs of bases together. Use the structures given in Table 21 of the Data Booklet.
Describe three characteristics of enzymes.
Compare the structures and chemical formulas of the two essential fatty acids linoleic acid and linolenic acid.
Pepsin is an enzyme, found in the stomach, that speeds up the breakdown of proteins. Iron is used to speed up the production of ammonia in the Haber process.
Describe the characteristics of an enzyme such as pepsin, and compare its catalytic behaviour to an inorganic catalyst such as iron.
Enzymes are affected by inhibitors. Lead ions are a non-competitive inhibitor, they have been linked to impaired mental functioning. Ritonavir® is a drug used to treat HIV and acts as a competitive inhibitor. Compare the action of lead ions and Ritonavir® on enzymes, and how they affect the initial rate of reaction of the enzyme with its substrate and the values of \({K_{\text{m}}}\) and \({V_{{\text{max}}}}\).
Linolenic acid (omega-3 fatty acid) is an essential fatty acid.
Calculate the iodine number for linolenic acid, \({{\text{C}}_{{\text{17}}}}{{\text{H}}_{{\text{29}}}}{\text{COOH (}}{{\text{M}}_{\text{r}}} = 278.48{\text{)}}\). The condensed structural formula of linolenic acid is given in table 22 of the data booklet.
The structures of some natural pigments and three preservatives are given in Table 22 of the Data Booklet.
When the flavylium cation is placed in alkaline solution the structure changes to the quinoidal base. Explain why the colour changes from red to blue.
Proteins are made of long chains of amino acids.
(i) Pepsin is a protein which functions as an enzyme in human stomachs. Describe the mechanism of the catalytic activity of an enzyme.
(ii) Discuss two differences in the catalytic action of an enzyme such as pepsin and an inorganic catalyst such as nickel metal.
A natural pigment found in cranberries can exist in two forms.
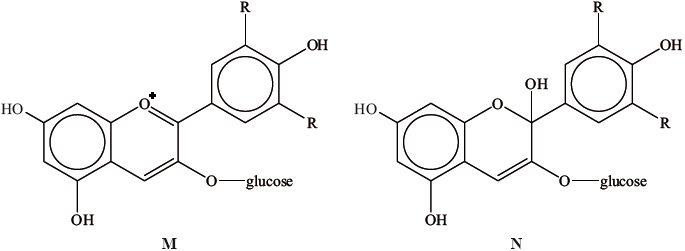
Explain, with reference to hybridization, which form is more likely to be coloured.
Vision is dependent on retinol (vitamin A) present in retina cells. Retinol is oxidized to the photosensitive chemical 11-cis-retinal and isomerizes to 11-trans-retinal on absorption of light.
Outline how the formation of 11-trans-retinal results in the generation of nerve signals to the brain.
State two differences in composition and one difference in structure between RNA and DNA.
Hemoglobin contains an iron ion that can bind to oxygen as part of the process of respiration.
Hemoglobin’s oxygen dissociation curve is shown at a given temperature. Sketch the curve on the graph at a higher temperature.
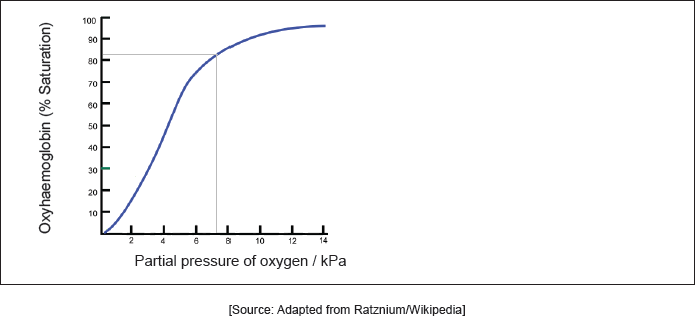
Outline two differences between normal hemoglobin and foetal hemoglobin.
Amino acids, shown in section 33 of the data booklet, can be combined to form polypeptides and proteins.
(i) Serine is a chiral amino acid. Draw both enantiomers of serine.
(ii) State the enantiomeric form of serine found in proteins.
Biological pigments include a variety of chemical structures with diverse functions.
The graph shows the conversion of hemoglobin to oxyhemoglobin.
Hb(aq) + 4O2(g) \( \rightleftharpoons \) Hb(O2)4(aq)
The partial pressure of oxygen gas, p(O2) is proportional to its concentration.
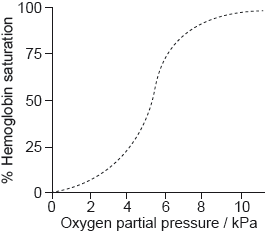
Explain the shape of the curve at low oxygen partial pressure up to about 5 kPa.
Sketch a graph on the axes above to show the effect of decreasing pH on the binding of oxygen to hemoglobin (the Bohr Effect).
Outline the effect of decreasing pH on the oxygen saturation of hemoglobin.
Anthocyanins are pigments that give colour to many flowers and fruits. The red colour of ripe strawberries is mainly due to the anthocyanin pigment whose structure is shown below.
Outline why this molecule absorbs visible light.
With reference to its chemical structure, outline whether this pigment is found in aqueous solution in the cells or in the lipid-based membranes.
A student investigated the ability of anthocyanins to act as pH indicators. He extracted juice from blackberries and used a UV-vis spectrophotometer to produce absorption spectra at different pH values. His results are shown below.
Deduce the colour of the juice at each pH, giving your reasoning. Use section 17 of the data booklet.
Glucokinase and hexokinase are both enzymes that catalyse the conversion of glucose to glucose-6-phosphate. The enzymes differ, however, in their affinity for the substrate, as shown in the graph below.
(i) Estimate the Km values of the two enzymes.
(ii) Suggest, with a reason, which enzyme will be more responsive to changes in the concentration of glucose in the blood.
(i) Outline what is meant by product inhibition as it applies to hexokinase.
(ii) Product inhibition of hexokinase does not affect its Km value. Using this information, deduce the type of binding site that the inhibitor attaches to.
Analysis of amino acid and protein concentration is a key area of biological research.
The titration curve of aqueous glycine zwitterions with aqueous sodium hydroxide is shown from pH 6.0 to 13.0. Refer to section 33 of the data booklet.
Deduce the pH range in which glycine is an effective buffer in basic solution.
Enzymes are biological catalysts.
The data shows the effect of substrate concentration, [S], on the rate, v, of an enzyme-catalysed reaction.
Determine the value of the Michaelis constant (Km) from the data. A graph is not required.
Outline the action of a non-competitive inhibitor on the enzyme-catalysed reaction.
The sequence of nitrogenous bases in DNA determines hereditary characteristics.
Calculate the mole percentages of cytosine, guanine and thymine in a double helical DNA structure if it contains 17% adenine by mole.
The graph of the rate of an enzyme-catalyzed reaction is shown below.
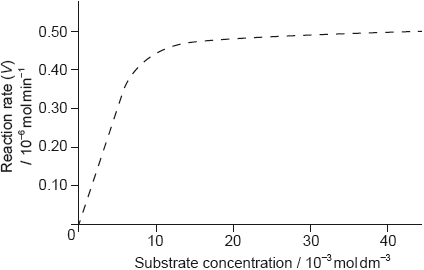
Determine the value of the Michaelis constant, Km, including units, from the graph.
Sketch a second graph on the same axes to show how the reaction rate varies when a competitive inhibitor is present.
Outline the significance of the value of Km.
Spinach is an excellent source of vitamins A and C.
Identify one structural characteristic in vitamins A and D which makes them more similar to each other than they are to vitamin C using section 35 of the data booklet.
The pigments from spinach were separated using chromatography. Identify Z by calculating its Rf value and using the data table.
Rf:
Z:
The stability of DNA is due to interactions of its hydrophilic and hydrophobic components.
Outline the interactions of the phosphate groups in DNA with water and with surrounding proteins (histones).
Enzymes play an important role in the functioning of our bodies.
The graph below shows a Michaelis–Menten plot for an enzyme. Sketch and label two curves on the graph below to show the effect of adding a competitive and non-competitive inhibitor.
Enzyme solutions are prepared in buffers. Determine the pH of a buffer solution containing 2.60×10−3moldm−3 ethanoic acid and 3.70×10−3moldm−3 sodium ethanoate. Refer to sections 1 and 21 of the data booklet.
An enzyme catalyses the conversion of succinate to fumarate ions in a cell, as part of the process of respiration.
The rate of the reaction was monitored and the following graph was plotted.
Determine the value of the Michaelis constant, Km, by annotating the graph.
The malonate ion acts as an inhibitor for the enzyme.
Suggest, on the molecular level, how the malonate ion is able to inhibit the enzyme.
Draw a curve on the graph above showing the effect of the presence of the malonate ion inhibitor on the rate of reaction.